Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures
- PMID: 20080548
- PMCID: PMC2798883
- DOI: 10.1073/pnas.0905481107
Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures
Abstract
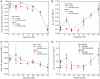
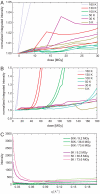
Radiation damage is the major impediment for obtaining structural information from biological samples by using ionizing radiation such as x-rays or electrons. The knowledge of underlying processes especially at cryogenic temperatures is still fragmentary, and a consistent mechanism has not been found yet. By using a combination of single-crystal x-ray diffraction, small-angle scattering, and qualitative and quantitative radiolysis experiments, we show that hydrogen gas, formed inside the sample during irradiation, rather than intramolecular bond cleavage between non-hydrogen atoms, is mainly responsible for the loss of high-resolution information and contrast in diffraction experiments and microscopy. The experiments that are presented in this paper cover a temperature range between 5 and 160 K and reveal that the commonly used temperature in x-ray crystallography of 100 K is not optimal in terms of minimizing radiation damage and thereby increasing the structural information obtainable in a single experiment. At 50 K, specific radiation damage to disulfide bridges is reduced by a factor of 4 compared to 100 K, and samples can tolerate a factor of 2.6 and 3.9 higher dose, as judged by the increase of R(free) values of elastase and cubic insulin crystals, respectively.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Radiation damage of protein crystals at cryogenic temperatures between 40 K and 150 K.J Synchrotron Radiat. 2002 Jul 1;9(Pt 4):198-201. doi: 10.1107/s0909049502008579. Epub 2002 Jun 30. J Synchrotron Radiat. 2002. PMID: 12091725
-
Quantifying X-ray radiation damage in protein crystals at cryogenic temperatures.Acta Crystallogr D Biol Crystallogr. 2006 Sep;62(Pt 9):1030-8. doi: 10.1107/S0907444906023869. Epub 2006 Aug 19. Acta Crystallogr D Biol Crystallogr. 2006. PMID: 16929104
-
Radiation damage to biological samples: still a pertinent issue.J Synchrotron Radiat. 2021 Sep 1;28(Pt 5):1278-1283. doi: 10.1107/S1600577521008845. Epub 2021 Sep 1. J Synchrotron Radiat. 2021. PMID: 34475277 Free PMC article.
-
Radiation damage to biological macromolecules∗.Curr Opin Struct Biol. 2023 Oct;82:102662. doi: 10.1016/j.sbi.2023.102662. Epub 2023 Aug 11. Curr Opin Struct Biol. 2023. PMID: 37573816 Review.
-
Radiation damage to protein specimens from electron beam imaging and diffraction: a mini-review of anti-damage approaches, with special reference to synchrotron X-ray crystallography.J Synchrotron Radiat. 2007 Jan;14(Pt 1):116-27. doi: 10.1107/S0909049506052307. Epub 2006 Dec 15. J Synchrotron Radiat. 2007. PMID: 17211078 Review.
Cited by
-
Identifying and quantifying radiation damage at the atomic level.J Synchrotron Radiat. 2015 Mar;22(2):201-12. doi: 10.1107/S1600577515002131. Epub 2015 Feb 14. J Synchrotron Radiat. 2015. PMID: 25723922 Free PMC article. Review.
-
Breaking the radiation damage limit with Cryo-SAXS.Biophys J. 2013 Jan 8;104(1):227-36. doi: 10.1016/j.bpj.2012.11.3817. Epub 2013 Jan 8. Biophys J. 2013. PMID: 23332075 Free PMC article.
-
1 kHz fixed-target serial crystallography using a multilayer monochromator and an integrating pixel detector.IUCrJ. 2019 Aug 17;6(Pt 5):927-937. doi: 10.1107/S205225251900914X. eCollection 2019 Sep 1. IUCrJ. 2019. PMID: 31576225 Free PMC article.
-
The domain swapping of human cystatin C induced by synchrotron radiation.Sci Rep. 2019 Jun 12;9(1):8548. doi: 10.1038/s41598-019-44811-1. Sci Rep. 2019. PMID: 31189973 Free PMC article.
-
Radiation damage in room-temperature data acquisition with the PILATUS 6M pixel detector.J Synchrotron Radiat. 2011 May;18(Pt 3):318-28. doi: 10.1107/S090904951100968X. Epub 2011 Apr 9. J Synchrotron Radiat. 2011. PMID: 21525639 Free PMC article.
References
-
- Henderson R. Cryo-protection of protein crystals against radiation damage in electron and x-ray diffraction. Proc Roy Soc Lond B Bio. 1990;241:6–8.
-
- Nave C. Radiation damage in protein crystallography. Radiat Phys Chem. 1995;45(3):483–490.
-
- Teng T-y, Moffat K. Primary radiation damage of protein crystals by an intense synchrotron x-ray beam. J Synchrotron Radiat. 2000;7(5):313–317. - PubMed
-
- Mozumder A, Magee JL. Model of tracks of ionizing radiations for radical reaction mechanisms. Radiat Res. 1966;28(2):203–214. - PubMed
-
- Ziaja B, London RA, Hajdu J. Unified model of secondary electron cascades in diamond. J Appl Phys. 2005;97(6):064905–064909.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

