Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons
- PMID: 20080551
- PMCID: PMC2824309
- DOI: 10.1073/pnas.0910302107
Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons
Abstract
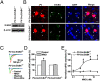
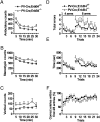
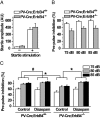
Neuregulin 1 (NRG1) is a trophic factor thought to play a role in neural development. Recent studies suggest that it may regulate neurotransmission, mechanisms of which remain elusive. Here we show that NRG1, via stimulating GABA release from interneurons, inhibits pyramidal neurons in the prefrontal cortex (PFC). Ablation of the NRG1 receptor ErbB4 in parvalbumin (PV)-positive interneurons prevented NRG1 from stimulating GABA release and from inhibiting pyramidal neurons. PV-ErbB4(-/-) mice exhibited schizophrenia-relevant phenotypes similar to those observed in NRG1 or ErbB4 null mutant mice, including hyperactivity, impaired working memory, and deficit in prepulse inhibition (PPI) that was ameliorated by diazepam, a GABA enhancer. These results indicate that NRG1 regulates the activity of pyramidal neurons by promoting GABA release from PV-positive interneurons, identifying a critical function of NRG1 in balancing brain activity. Because both NRG1 and ErbB4 are susceptibility genes of schizophrenia, our study provides insight into potential pathogenic mechanisms of schizophrenia and suggests that PV-ErbB4(-/-) mice may serve as a model in the study of this and relevant brain disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21818-23. doi: 10.1073/pnas.1010669107. Epub 2010 Nov 24. Proc Natl Acad Sci U S A. 2010. PMID: 21106764 Free PMC article.
-
Regulation of spine formation by ErbB4 in PV-positive interneurons.J Neurosci. 2013 Dec 4;33(49):19295-303. doi: 10.1523/JNEUROSCI.2090-13.2013. J Neurosci. 2013. PMID: 24305825 Free PMC article.
-
Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling.Nature. 2010 Apr 29;464(7293):1376-80. doi: 10.1038/nature08928. Epub 2010 Apr 14. Nature. 2010. PMID: 20393464
-
Neuregulin 1: an intriguing therapeutic target for neurodevelopmental disorders.Transl Psychiatry. 2020 Jun 16;10(1):190. doi: 10.1038/s41398-020-00868-5. Transl Psychiatry. 2020. PMID: 32546684 Free PMC article. Review.
-
Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway.Psychopharmacology (Berl). 2013 Mar;226(2):201-15. doi: 10.1007/s00213-013-3003-2. Epub 2013 Feb 7. Psychopharmacology (Berl). 2013. PMID: 23389757 Review.
Cited by
-
Neuregulin 1 Controls Glutamate Uptake by Up-regulating Excitatory Amino Acid Carrier 1 (EAAC1).J Biol Chem. 2015 Aug 14;290(33):20233-44. doi: 10.1074/jbc.M114.591867. Epub 2015 Jun 19. J Biol Chem. 2015. PMID: 26092725 Free PMC article.
-
Modeling schizophrenia using induced pluripotent stem cell-derived and fibroblast-induced neurons.Schizophr Bull. 2013 Jan;39(1):4-10. doi: 10.1093/schbul/sbs127. Epub 2012 Nov 19. Schizophr Bull. 2013. PMID: 23172000 Free PMC article.
-
Abnormal behavior in mice mutant for the Disc1 binding partner, Dixdc1.Transl Psychiatry. 2011 Sep 27;1(9):e43. doi: 10.1038/tp.2011.41. Transl Psychiatry. 2011. PMID: 22832659 Free PMC article.
-
Preferential expression of SCN1A in GABAergic neurons improves survival and epileptic phenotype in a mouse model of Dravet syndrome.J Mol Med (Berl). 2023 Dec;101(12):1587-1601. doi: 10.1007/s00109-023-02383-8. Epub 2023 Oct 11. J Mol Med (Berl). 2023. PMID: 37819378 Free PMC article.
-
Antibody-mediated stabilization of NRG1 induces behavioral and electrophysiological alterations in adult mice.Sci Rep. 2018 May 29;8(1):8239. doi: 10.1038/s41598-018-26492-4. Sci Rep. 2018. PMID: 29844389 Free PMC article.
References
-
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. - PubMed
-
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

