Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles
- PMID: 20080552
- PMCID: PMC2824286
- DOI: 10.1073/pnas.0914140107
Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles
Abstract
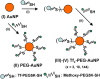
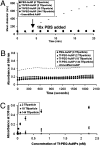
PEGylated gold nanoparticles are decorated with various amounts of human transferrin (Tf) to give a series of Tf-targeted particles with near-constant size and electrokinetic potential. The effects of Tf content on nanoparticle tumor targeting were investigated in mice bearing s.c. Neuro2A tumors. Quantitative biodistributions of the nanoparticles 24 h after i.v. tail-vein injections show that the nanoparticle accumulations in the tumors and other organs are independent of Tf. However, the nanoparticle localizations within a particular organ are influenced by the Tf content. In tumor tissue, the content of targeting ligands significantly influences the number of nanoparticles localized within the cancer cells. In liver tissue, high Tf content leads to small amounts of the nanoparticles residing in hepatocytes, whereas most nanoparticles remain in nonparenchymal cells. These results suggest that targeted nanoparticles can provide greater intracellular delivery of therapeutic agents to the cancer cells within solid tumors than their nontargeted analogs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

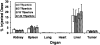


References
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. - PubMed
-
- Dreher MR, et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. - PubMed
-
- Lopes de Menezes DE, Pilarski LM, Allen TM. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res. 1998;58:3320–3330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

