Cooperative nanomaterial system to sensitize, target, and treat tumors
- PMID: 20080556
- PMCID: PMC2824295
- DOI: 10.1073/pnas.0909565107
Cooperative nanomaterial system to sensitize, target, and treat tumors
Abstract
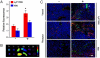
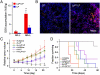
A significant barrier to the clinical translation of systemically administered therapeutic nanoparticles is their tendency to be removed from circulation by the mononuclear phagocyte system. The addition of a targeting ligand that selectively interacts with cancer cells can improve the therapeutic efficacy of nanomaterials, although these systems have met with only limited success. Here, we present a cooperative nanosystem consisting of two discrete nanomaterials. The first component is gold nanorod (NR) "activators" that populate the porous tumor vessels and act as photothermal antennas to specify tumor heating via remote near-infrared laser irradiation. We find that local tumor heating accelerates the recruitment of the second component: a targeted nanoparticle consisting of either magnetic nanoworms (NW) or doxorubicin-loaded liposomes (LP). The targeting species employed in this work is a cyclic nine-amino acid peptide LyP-1 (Cys-Gly-Asn-Lys-Arg-Thr-Arg-Gly-Cys) that binds to the stress-related protein, p32, which we find to be upregulated on the surface of tumor-associated cells upon thermal treatment. Mice containing xenografted MDA-MB-435 tumors that are treated with the combined NR/LyP-1LP therapeutic system display significant reductions in tumor volume compared with individual nanoparticles or untargeted cooperative system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

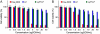
References
-
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. - PubMed
-
- Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–7248. - PubMed
-
- Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WCW. Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv Mater. 2008;20:3832–3838.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

