Bioartificial matrices for therapeutic vascularization
- PMID: 20080569
- PMCID: PMC2840448
- DOI: 10.1073/pnas.0905447107
Bioartificial matrices for therapeutic vascularization
Abstract
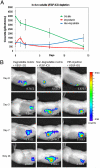
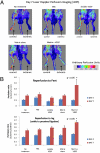
Therapeutic vascularization remains a significant challenge in regenerative medicine applications. Whether the goal is to induce vascular growth in ischemic tissue or scale up tissue-engineered constructs, the ability to induce the growth of patent, stable vasculature is a critical obstacle. We engineered polyethylene glycol-based bioartificial hydrogel matrices presenting protease-degradable sites, cell-adhesion motifs, and growth factors to induce the growth of vasculature in vivo. Compared to injection of soluble VEGF, these matrices delivered sustained in vivo levels of VEGF over 2 weeks as the matrix degraded. When implanted subcutaneously in rats, degradable constructs containing VEGF and arginine-glycine-aspartic acid tripeptide induced a significant number of vessels to grow into the implant at 2 weeks with increasing vessel density at 4 weeks. The mechanism of enhanced vascularization is likely cell-demanded release of VEGF, as the hydrogels may degrade substantially within 2 weeks. In a mouse model of hind-limb ischemia, delivery of these matrices resulted in significantly increased rate of reperfusion. These results support the application of engineered bioartificial matrices to promote vascularization for directed regenerative therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Peripheral Artery Disease Quick Facts. American Heart Association; 2009.
-
- Heart Disease and Stroke Statistics—2009 Update. American Heart Association; 2009.
-
- van Weel V, van Tongeren RB, van Hinsbergh VWM, van Bockel JH, Quax PHA. Vascular growth in ischemic limbs: A review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–597. - PubMed
-
- Hummers LK, Hall A, Wigley FM, Simons M. Evidence for abnormal angiogenesis in scleroderma patients. Arthritis and Rheum. 2004;50:S630–S631.
-
- Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: Protein therapy. Cardiovasc Res. 2005;65:649–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

