Live-cell imaging of photosystem II antenna dissociation during state transitions
- PMID: 20080575
- PMCID: PMC2836652
- DOI: 10.1073/pnas.0908808107
Live-cell imaging of photosystem II antenna dissociation during state transitions
Abstract
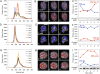
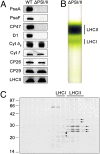
Plants and green algae maintain efficient photosynthesis under changing light environments by adjusting their light-harvesting capacity. It has been suggested that energy redistribution is brought about by shuttling the light-harvesting antenna complex II (LHCII) between photosystem II (PSII) and photosystem I (PSI) (state transitions), but such molecular remodeling has never been demonstrated in vivo. Here, using chlorophyll fluorescence lifetime imaging microscopy, we visualized phospho-LHCII dissociation from PSII in live cells of the green alga Chlamydomonas reinhardtii. Induction of energy redistribution in wild-type cells led to an increase in, and spreading of, a 250-ps lifetime chlorophyll fluorescence component, which was not observed in the stt7 mutant incapable of state transitions. The 250-ps component was also the dominant component in a mutant containing the light-harvesting antenna complexes but no photosystems. The appearance of the 250-ps component was accompanied by activation of LHCII phosphorylation, supporting the visualization of phospho-LHCII dissociation. Possible implications of the unbound phospho-LHCII on energy dissipation are discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
The photonic "smart grid" of the chloroplast in action.Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):2729-30. doi: 10.1073/pnas.0914429107. Epub 2010 Feb 5. Proc Natl Acad Sci U S A. 2010. PMID: 20139299 Free PMC article. No abstract available.
References
-
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969;172:242–251. - PubMed
-
- Bonaventura C, Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189:366–383. - PubMed
-
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature. 1981;291:25–29.
-
- Wollman F-A, Lemaire C. Studies on kinase-controlled state transitions in photosystem II and b6 f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim Biophys Acta. 1988;933:85–94.
-
- Bellafiore S, Barneche F, Peltier G, Rochaix J-D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature. 2005;433:892–895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

