Light changes the atmospheric reactivity of soot
- PMID: 20080580
- PMCID: PMC2872373
- DOI: 10.1073/pnas.0908341107
Light changes the atmospheric reactivity of soot
Abstract
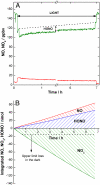
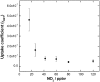
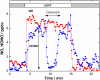
Soot particles produced by incomplete combustion processes are one of the major components of urban air pollution. Chemistry at their surfaces lead to the heterogeneous conversion of several key trace gases; for example NO(2) interacts with soot and is converted into HONO, which rapidly photodissociates to form OH in the troposphere. In the dark, soot surfaces are rapidly deactivated under atmospheric conditions, leading to the current understanding that soot chemistry affects tropospheric chemical composition only in a minor way. We demonstrate here that the conversion of NO(2) to HONO on soot particles is drastically enhanced in the presence of artificial solar radiation, and leads to persistent reactivity over long periods. Soot photochemistry may therefore be a key player in urban air pollution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


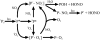

Similar articles
-
Heterogeneous reaction of NO2 on diesel soot particles.Environ Sci Technol. 2001 Jun 1;35(11):2191-9. doi: 10.1021/es000207s. Environ Sci Technol. 2001. PMID: 11414018
-
Mineral oxides change the atmospheric reactivity of soot: NO2 uptake under dark and UV irradiation conditions.J Phys Chem A. 2013 Dec 5;117(48):12897-911. doi: 10.1021/jp407914f. Epub 2013 Nov 22. J Phys Chem A. 2013. PMID: 24188183
-
Evidence of aerosols as a media for rapid daytime HONO production over China.Environ Sci Technol. 2014 Dec 16;48(24):14386-91. doi: 10.1021/es504163z. Epub 2014 Nov 26. Environ Sci Technol. 2014. PMID: 25401515
-
Sources of atmospheric nitrous acid: state of the science, current research needs, and future prospects.J Air Waste Manag Assoc. 2014 Nov;64(11):1232-50. doi: 10.1080/10962247.2014.952846. J Air Waste Manag Assoc. 2014. PMID: 25509545 Review.
-
Hazardous components and health effects of atmospheric aerosol particles: reactive oxygen species, soot, polycyclic aromatic compounds and allergenic proteins.Free Radic Res. 2012 Aug;46(8):927-39. doi: 10.3109/10715762.2012.663084. Epub 2012 Apr 23. Free Radic Res. 2012. PMID: 22300277 Review.
Cited by
-
Diesel soot photooxidation enhances the heterogeneous formation of H2SO4.Nat Commun. 2022 Sep 12;13(1):5364. doi: 10.1038/s41467-022-33120-3. Nat Commun. 2022. PMID: 36097270 Free PMC article.
-
Soil surface acidity plays a determining role in the atmospheric-terrestrial exchange of nitrous acid.Proc Natl Acad Sci U S A. 2014 Dec 30;111(52):18472-7. doi: 10.1073/pnas.1418545112. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512517 Free PMC article.
-
The Impact of Agroecosystems on Nitrous Acid (HONO) Emissions during Spring and Autumn in the North China Plain.Toxics. 2024 Apr 30;12(5):331. doi: 10.3390/toxics12050331. Toxics. 2024. PMID: 38787110 Free PMC article.
-
Role of elemental carbon in the photochemical aging of soot.Proc Natl Acad Sci U S A. 2018 Jul 24;115(30):7717-7722. doi: 10.1073/pnas.1804481115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987028 Free PMC article.
-
Enhancing soot oxidation using microtextured surfaces.Sci Rep. 2024 Feb 21;14(1):4247. doi: 10.1038/s41598-024-54320-5. Sci Rep. 2024. PMID: 38378782 Free PMC article.
References
-
- Forster P, et al. Changes in Atmospheric Constituents and in Radiative Forcing. 2007 http://ipcc-wg1.ucar.edu/wg1/wg1-report.html.
-
- Jacobson MZ. Strong radiative heating due to the mixing state of black carbon in atmospheric aerosols. Nature. 2001;409(6821):695–697. - PubMed
-
- Akhter MS, Chughtai AR, Smith DM. The structure of hexane soot I: Spectroscopic Studies. Appl Spectrosc. 1985;39(1):143–153.
-
- Akhter MS, Chughtai AR, Smith DM. The structure of hexane soot II: Extraction studies. Appl Spectrosc. 1985;39(1):154–167.
-
- IARC. Overall evaluations of carcinogenicity: An updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl . 1987;7:1–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

