Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion
- PMID: 20080584
- PMCID: PMC2824400
- DOI: 10.1073/pnas.0911996107
Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion
Abstract
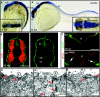
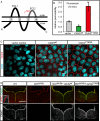
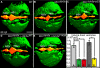
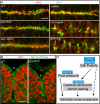
Lumen expansion driven by hydrostatic pressure occurs during many morphogenetic processes. Although it is well established that members of the Claudin family of transmembrane tight junction proteins determine paracellular tightness within epithelial/endothelial barrier systems, functional evidence for their role in the morphogenesis of lumenized organs has been scarce. Here, we identify Claudin5a as a core component of an early cerebral-ventricular barrier system that is required for ventricular lumen expansion in the zebrafish embryonic brain before the establishment of the embryonic blood-brain barrier. Loss of Claudin5a or expression of a tight junction-opening Claudin5a mutant reduces brain ventricular volume expansion without disrupting the polarized organization of the neuroepithelium. Perfusion experiments with the electron-dense small molecule lanthanum nitrate reveal that paracellular tightness of the cerebral-ventricular barrier decreases upon loss of Claudin5a. Genetic analyses show that the apical neuroepithelial localization of Claudin5a depends on epithelial cell polarity and provide evidence for concerted activities between Claudin5a and Na(+),K(+)-ATPase during luminal expansion of brain ventricles. These data establish an essential role of a barrier-forming Claudin in ventricular lumen expansion, thereby contributing to brain morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057–2067. - PubMed
-
- Jeong JY, et al. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 2008;75:619–628. - PubMed
-
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

