Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space
- PMID: 20080597
- PMCID: PMC2824412
- DOI: 10.1073/pnas.0913626107
Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space
Abstract
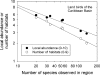
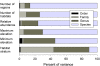
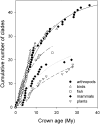
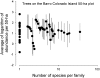
The population component of a species' niche corresponds to the distribution of individuals across environments within a region. As evolutionary clades of species diversify, they presumably fill niche space, and, consequently, the rate of increase in species numbers slows. Total niche space and species numbers appear to be relatively stable over long periods, and so an increase in the species richness of one clade must be balanced by decrease in others. However, in several analyses, the total population niche space occupied per clade is independent of the number of species, suggesting that species in more diverse clades overlap more in niche space. This overlap appears to be accommodated by variation in the populations of each species, including their absence, within suitable niche space. I suggest that the uneven filling of niche space results from localized outcomes of the dynamic coevolutionary interactions of populations with their pathogens or other antagonists. Furthermore, I speculate that relationships with pathogens might constrain diversification if pathogen diversity increased with host diversity and resulted in more frequent host switching and emergent disease. Many indirect observations are consistent with these scenarios. However, the postulated influence of pathogens on the filling of niche space and diversification of clades primarily highlights our lack of knowledge concerning the space and time dimensions of coevolutionary interactions and their influence on population distribution and species diversification.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Chase JM, Leibold MA. Ecological Niches. Linking Classical and Contemporary Approaches. Chicago: University of Chicago Press; 2003.
-
- Hutchinson GE. Concluding remarks. Cold Spring Harb Symp Quant Biol. 1957;22:415–427.
-
- McPeek MA. The ecological dynamics of clade diversification and community assembly. Am Nat. 2008;172:E270–E284. - PubMed
-
- Simpson GG. The Major Features of Evolution. New York: Columbia University Press; 1953.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

