Copper-free click chemistry in living animals
- PMID: 20080615
- PMCID: PMC2836626
- DOI: 10.1073/pnas.0911116107
Copper-free click chemistry in living animals
Abstract
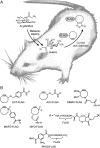
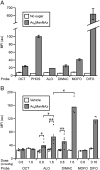
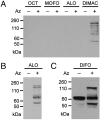
Chemical reactions that enable selective biomolecule labeling in living organisms offer a means to probe biological processes in vivo. Very few reactions possess the requisite bioorthogonality, and, among these, only the Staudinger ligation between azides and triarylphosphines has been employed for direct covalent modification of biomolecules with probes in the mouse, an important model organism for studies of human disease. Here we explore an alternative bioorthogonal reaction, the 1,3-dipolar cycloaddition of azides and cyclooctynes, also known as "Cu-free click chemistry," for labeling biomolecules in live mice. Mice were administered peracetylated N-azidoacetylmannosamine (Ac(4)ManNAz) to metabolically label cell-surface sialic acids with azides. After subsequent injection with cyclooctyne reagents, glycoconjugate labeling was observed on isolated splenocytes and in a variety of tissues including the intestines, heart, and liver, with no apparent toxicity. The cyclooctynes tested displayed various labeling efficiencies that likely reflect the combined influence of intrinsic reactivity and bioavailability. These studies establish Cu-free click chemistry as a bioorthogonal reaction that can be executed in the physiologically relevant context of a mouse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Peters LL, et al. The mouse as a model for human biology: A resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. - PubMed
-
- Ohtsubo K, et al. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. - PubMed
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Kayser H, et al. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 1992;267:16934–16938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

