Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate
- PMID: 20080618
- PMCID: PMC2836628
- DOI: 10.1073/pnas.0911185107
Identical phosphatase mechanisms achieved through distinct modes of binding phosphoprotein substrate
Abstract
Two-component signal transduction systems are widespread in prokaryotes and control numerous cellular processes. Extensive investigation of sensor kinase and response regulator proteins from many two-component systems has established conserved sequence, structural, and mechanistic features within each family. In contrast, the phosphatases which catalyze hydrolysis of the response regulator phosphoryl group to terminate signal transduction are poorly understood. Here we present structural and functional characterization of a representative of the CheC/CheX/FliY phosphatase family. The X-ray crystal structure of Borrelia burgdorferi CheX complexed with its CheY3 substrate and the phosphoryl analogue reveals a binding orientation between a response regulator and an auxiliary protein different from that shared by every previously characterized example. The surface of CheY3 containing the phosphoryl group interacts directly with a long helix of CheX which bears the conserved (E - X(2) - N) motif. Conserved CheX residues Glu96 and Asn99, separated by a single helical turn, insert into the CheY3 active site. Structural and functional data indicate that CheX Asn99 and CheY3 Thr81 orient a water molecule for hydrolytic attack. The catalytic residues of the CheX.CheY3 complex are virtually superimposable on those of the Escherichia coli CheZ phosphatase complexed with CheY, even though the active site helices of CheX and CheZ are oriented nearly perpendicular to one other. Thus, evolution has found two structural solutions to achieve the same catalytic mechanism through different helical spacing and side chain lengths of the conserved acid/amide residues in CheX and CheZ.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 . (A) Ribbon representation of the asymmetric unit. CheX is cyan and CheY3 is green. CheY3 Asp79 (orange),
. (A) Ribbon representation of the asymmetric unit. CheX is cyan and CheY3 is green. CheY3 Asp79 (orange),  (purple), CheX Glu96 (red, Upper), and CheX Asn99 (red, Lower) are in stick representation. Mg2+ is a magenta sphere. (B).Overlay of B. burgdorferi CheX (cyan) and T. maritima CheX chain B (pdb 1XKO, tan). Regions of B. burgdorferi CheX with high disorder (Cα thermal B-factors > 70) are blue. B. burgdorferi CheX residues 37–40 are sketched as blue dots. T. maritima CheX residues corresponding to the disordered regions of B. burgdorferi CheX are red.
(purple), CheX Glu96 (red, Upper), and CheX Asn99 (red, Lower) are in stick representation. Mg2+ is a magenta sphere. (B).Overlay of B. burgdorferi CheX (cyan) and T. maritima CheX chain B (pdb 1XKO, tan). Regions of B. burgdorferi CheX with high disorder (Cα thermal B-factors > 70) are blue. B. burgdorferi CheX residues 37–40 are sketched as blue dots. T. maritima CheX residues corresponding to the disordered regions of B. burgdorferi CheX are red.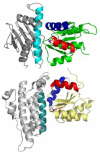
 (pdb 2R25). For
(pdb 2R25). For  (Upper), CheX is gray except for α1′, which is cyan. CheY3 is green except α1 is blue and α5 is red. For
(Upper), CheX is gray except for α1′, which is cyan. CheY3 is green except α1 is blue and α5 is red. For  (Lower), YPD1 is gray except for αC (teal). SLN1-R1 is pale yellow except α1 is blue and α5 is red.
(Lower), YPD1 is gray except for αC (teal). SLN1-R1 is pale yellow except α1 is blue and α5 is red.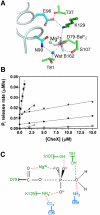
 persist in the transition state. One of several possible hydrogen bonding arrangements between CheY3 T81 or CheX N99 and the water is shown. Residues from CheY3 (green) and CheX (cyan) are indicated.
persist in the transition state. One of several possible hydrogen bonding arrangements between CheY3 T81 or CheX N99 and the water is shown. Residues from CheY3 (green) and CheX (cyan) are indicated.
 and E. coli
and E. coli  (pdb 1KMI) with superposition of CheY3 (green) and CheY (light green). CheX is cyan and CheZ is orange. CheX Glu96 (Left) and Asn99 (Right) are in cyan sticks. CheZ Asp143 (Left) and Gln147 (Right) are in orange sticks. The
(pdb 1KMI) with superposition of CheY3 (green) and CheY (light green). CheX is cyan and CheZ is orange. CheX Glu96 (Left) and Asn99 (Right) are in cyan sticks. CheZ Asp143 (Left) and Gln147 (Right) are in orange sticks. The  moiety is in purple sticks. (B) Close-up of region within the indicated square from Panel (A). Select active site groups from B. burgdorferi CheY3 (green) or E. coli CheY (light green) are shown. The residues are sticks and the divalent cations (Mg2+ for CheY3 and Mn2+ for CheY) are spheres.
moiety is in purple sticks. (B) Close-up of region within the indicated square from Panel (A). Select active site groups from B. burgdorferi CheY3 (green) or E. coli CheY (light green) are shown. The residues are sticks and the divalent cations (Mg2+ for CheY3 and Mn2+ for CheY) are spheres.Similar articles
-
Structure and activity of the flagellar rotor protein FliY: a member of the CheC phosphatase family.J Biol Chem. 2013 May 10;288(19):13493-502. doi: 10.1074/jbc.M112.445171. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532838 Free PMC article.
-
CheX is a phosphorylated CheY phosphatase essential for Borrelia burgdorferi chemotaxis.J Bacteriol. 2005 Dec;187(23):7963-9. doi: 10.1128/JB.187.23.7963-7969.2005. J Bacteriol. 2005. PMID: 16291669 Free PMC article.
-
Structure and function of an unusual family of protein phosphatases: the bacterial chemotaxis proteins CheC and CheX.Mol Cell. 2004 Nov 19;16(4):563-74. doi: 10.1016/j.molcel.2004.10.018. Mol Cell. 2004. PMID: 15546616
-
Auxiliary phosphatases in two-component signal transduction.Curr Opin Microbiol. 2010 Apr;13(2):177-83. doi: 10.1016/j.mib.2010.01.004. Epub 2010 Feb 3. Curr Opin Microbiol. 2010. PMID: 20133180 Free PMC article. Review.
-
Negative control in two-component signal transduction by transmitter phosphatase activity.Mol Microbiol. 2011 Oct;82(2):275-86. doi: 10.1111/j.1365-2958.2011.07829.x. Epub 2011 Sep 29. Mol Microbiol. 2011. PMID: 21895797 Free PMC article. Review.
Cited by
-
Phosphorylation chemistry of the Bordetella PlrSR TCS and its contribution to bacterial persistence in the lower respiratory tract.Mol Microbiol. 2023 Feb;119(2):174-190. doi: 10.1111/mmi.15019. Epub 2023 Jan 16. Mol Microbiol. 2023. PMID: 36577696 Free PMC article.
-
Nonconserved active site residues modulate CheY autophosphorylation kinetics and phosphodonor preference.Biochemistry. 2013 Apr 2;52(13):2262-73. doi: 10.1021/bi301654m. Epub 2013 Mar 19. Biochemistry. 2013. PMID: 23458124 Free PMC article.
-
The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi.Front Cell Infect Microbiol. 2014 May 1;4:56. doi: 10.3389/fcimb.2014.00056. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24822172 Free PMC article. Review.
-
Bordetella PlrSR regulatory system controls BvgAS activity and virulence in the lower respiratory tract.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1519-E1527. doi: 10.1073/pnas.1609565114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167784 Free PMC article.
-
Structural insights into flagellar stator-rotor interactions.Elife. 2019 Jul 17;8:e48979. doi: 10.7554/eLife.48979. Elife. 2019. PMID: 31313986 Free PMC article.
References
-
- Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. - PubMed
-
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. - PubMed
-
- Calva E, Oropeza R. Two-component signal transduction systems, environmental signals, and virulence. Microb Ecol. 2006;51:166–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

