Observations of aminium salts in atmospheric nanoparticles and possible climatic implications
- PMID: 20080626
- PMCID: PMC2872393
- DOI: 10.1073/pnas.0912127107
Observations of aminium salts in atmospheric nanoparticles and possible climatic implications
Abstract
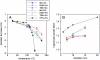
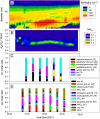
We present laboratory studies and field observations that explore the role of aminium salt formation in atmospheric nanoparticle growth. These measurements were performed using the Thermal Desorption Chemical Ionization Mass Spectrometer (TDCIMS) and Ultrafine Hygroscopicity Tandem Differential Mobility Analyzers. Laboratory measurements of alkylammonium-carboxylate salt nanoparticles show that these particles exhibit lower volatilities and only slightly lower hygroscopicities than ammonium sulfate nanoparticles. TDCIMS measurements of these aminium salts showed that the protonated amines underwent minimal decomposition during analysis, with detection sensitivities comparable to those of organic and inorganic deprotonated acids. TDCIMS observations made of a new particle formation event in an urban site in Tecamac, Mexico, clearly indicate the presence of protonated amines in 8-10 nm diameter particles accounting for about 47% of detected positive ions; 13 nm particles were hygroscopic with an average 90% RH growth factor of 1.42. Observations of a new particle formation event in a remote forested site in Hyytiälä, Finland, show the presence of aminium ions with deprotonated organic acids; 23% of the detected positive ions during this event are attributed to aminium salts while 10 nm particles had an average 90% RH growth factor of 1.27. Similar TDCIMS observations during events in Atlanta and in the vicinity of Boulder, Colorado, show that aminium salts accounted for 10-35% of detected positive ions. We conclude that aminium salts contribute significantly to nanoparticle growth and must be accounted for in models to accurately predict the impact of new particle formation on climate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Composition of Ultrafine Particles in Urban Beijing: Measurement Using a Thermal Desorption Chemical Ionization Mass Spectrometer.Environ Sci Technol. 2021 Mar 2;55(5):2859-2868. doi: 10.1021/acs.est.0c06053. Epub 2021 Feb 12. Environ Sci Technol. 2021. PMID: 33577293
-
Thermal characterization of aminium nitrate nanoparticles.J Phys Chem A. 2011 Oct 27;115(42):11671-7. doi: 10.1021/jp204957k. Epub 2011 Sep 30. J Phys Chem A. 2011. PMID: 21910406
-
Measurements of hygroscopicity and volatility of atmospheric ultrafine particles during ultrafine particle formation events at urban, industrial, and coastal sites.Environ Sci Technol. 2009 Sep 1;43(17):6710-6. doi: 10.1021/es900398q. Environ Sci Technol. 2009. PMID: 19764239
-
Mass spectrometry of atmospheric aerosols--recent developments and applications. Part II: On-line mass spectrometry techniques.Mass Spectrom Rev. 2012 Jan-Feb;31(1):17-48. doi: 10.1002/mas.20330. Epub 2011 Mar 29. Mass Spectrom Rev. 2012. PMID: 21449003 Review.
-
Continuous and semicontinuous monitoring techniques for particulate matter mass and chemical components: a synthesis of findings from EPA's Particulate Matter Supersites Program and related studies.J Air Waste Manag Assoc. 2008 Feb;58(2):164-95. doi: 10.3155/1047-3289.58.2.164. J Air Waste Manag Assoc. 2008. PMID: 18318336 Review.
Cited by
-
The future of airborne sulfur-containing particles in the absence of fossil fuel sulfur dioxide emissions.Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13514-9. doi: 10.1073/pnas.1510743112. Epub 2015 Oct 19. Proc Natl Acad Sci U S A. 2015. PMID: 26483454 Free PMC article.
-
The effect of acid-base clustering and ions on the growth of atmospheric nano-particles.Nat Commun. 2016 May 20;7:11594. doi: 10.1038/ncomms11594. Nat Commun. 2016. PMID: 27197574 Free PMC article.
-
Effect of inorganic salts on the volatility of organic acids.Environ Sci Technol. 2014 Dec 2;48(23):13718-26. doi: 10.1021/es5033103. Epub 2014 Nov 19. Environ Sci Technol. 2014. PMID: 25369247 Free PMC article.
-
Dimethylamine as a major alkyl amine species in particles and cloud water: Observations in semi-arid and coastal regions.Atmos Environ (1994). 2015 Dec;122:250-258. doi: 10.1016/j.atmosenv.2015.09.061. Atmos Environ (1994). 2015. PMID: 26807039 Free PMC article.
-
Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation.Nature. 2011 Aug 24;476(7361):429-33. doi: 10.1038/nature10343. Nature. 2011. PMID: 21866156
References
-
- Kulmala M, et al. Formation and growth rates of ultrafine atmospheric particles: A review of observations. J Aerosol Sci. 2004;35:143–175.
-
- Spracklen DV, et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophys Res Lett. 2008;35:L06808. doi: 10.1029/2007GL033038. - DOI
-
- Kuang C, McMurry PH, McCormick AV. Determination of cloud condensation nuclei production from measured new particle formation events. Geophys Res Lett. 2009;36:L09822. doi: 10.1029/2009GL037584. - DOI
-
- Iida K, Stolzenburg MR, McMurry PH, Smith JN. Estimating nanoparticle growth rates from size-dependent charged fractions—Analysis of new particle formation events in Mexico City. J Geophys Res Atmos. 2008;113:D05207. doi: 10.1029/2007JD009260. - DOI
-
- Eisele FL, Tanner DJ. Measurement of the gas phase concentration of H2SO4 and methane sulfonic acid and estimates of H2SO4 production and loss in the atmosphere. J Geophys Res. 1993;98:9001–9010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

