Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders
- PMID: 20080669
- PMCID: PMC2836639
- DOI: 10.1073/pnas.0904055107
Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders
Abstract
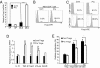
The beneficial effects of probiotics have been described in many diseases, but the mechanism by which they modulate the immune system is poorly understood. In this study, we identified a mixture of probiotics that up-regulates CD4(+)Foxp3(+) regulatory T cells (Tregs). Administration of the probiotics mixture induced both T-cell and B-cell hyporesponsiveness and down-regulated T helper (Th) 1, Th2, and Th17 cytokines without apoptosis induction. It also induced generation of CD4(+)Foxp3(+) Tregs from the CD4(+)CD25(-) population and increased the suppressor activity of naturally occurring CD4(+)CD25(+) Tregs. Conversion of T cells into Foxp3(+) Tregs is directly mediated by regulatory dendritic cells (rDCs) that express high levels of IL-10, TGF-beta, COX-2, and indoleamine 2,3-dioxygenase. Administration of probiotics had therapeutical effects in experimental inflammatory bowel disease, atopic dermatitis, and rheumatoid arthritis. The therapeutical effect of the probiotics is associated with enrichment of CD4(+)Foxp3(+) Tregs in the inflamed regions. Collectively, the administration of probiotics that enhance the generation of rDCs and Tregs represents an applicable treatment of inflammatory immune disorders.
Figures
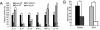


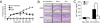

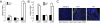
References
-
- Braat H, et al. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80:1618–1625. - PubMed
-
- So J-S, et al. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol Immunol. 2008;46:172–180. - PubMed
-
- So J-S, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008;45:2690–2699. - PubMed
-
- Sudo N, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–1116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

