UBR2 mediates transcriptional silencing during spermatogenesis via histone ubiquitination
- PMID: 20080676
- PMCID: PMC2836623
- DOI: 10.1073/pnas.0910267107
UBR2 mediates transcriptional silencing during spermatogenesis via histone ubiquitination
Abstract
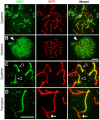
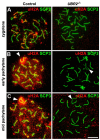
Ubiquitination of histones provides an important mechanism regulating chromatin remodeling and gene expression. Recent studies have revealed ubiquitin ligases involved in histone ubiquitination, yet the responsible enzymes and the function of histone ubiquitination in spermatogenesis remain unclear. We have previously shown that mice lacking the ubiquitin ligase UBR2, one of the recognition E3 components of the N-end rule proteolytic pathway, are infertile associated with meiotic arrest at prophase I. We here show that UBR2 localizes to meiotic chromatin regions, including unsynapsed axial elements linked to chromatin inactivation, and mediates transcriptional silencing via the ubiquitination of histone H2A. UBR2 interacts with the ubiquitin conjugating enzyme HR6B and its substrate H2A and promotes the HR6B-H2A interaction and the HR6B-to-H2A transfer of ubiquitin. UBR2 and ubiquitinated H2A (uH2A) spatiotemporally mark meiotic chromatin regions subject to transcriptional silencing, and UBR2-deficient spermatocytes fail to induce the ubiquitination of H2A during meiosis. UBR2-deficient spermatocytes are profoundly impaired in chromosome-wide transcriptional silencing of genes linked to unsynapsed axes of the X and Y chromosomes. Our findings suggest that insufficiency in UBR2-dependent histone ubiquitination triggers a pachytene checkpoint system, providing a new insight into chromatin remodeling and gene expression regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Tasaki T, Kwon YT. The mammalian N-end rule pathway: New insights into its components and physiological roles. Trends Biochem Sci. 2007;32:520–528. - PubMed
-
- Kwon YT, et al. An essential role of N-terminal arginylation in cardiovascular development. Science. 2002;297:96–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

