Thioredoxin suppresses microscopic hopping of T7 DNA polymerase on duplex DNA
- PMID: 20080681
- PMCID: PMC2836631
- DOI: 10.1073/pnas.0912664107
Thioredoxin suppresses microscopic hopping of T7 DNA polymerase on duplex DNA
Abstract
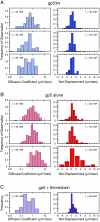
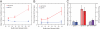
The DNA polymerases involved in DNA replication achieve high processivity of nucleotide incorporation by forming a complex with processivity factors. A model system for replicative DNA polymerases, the bacteriophage T7 DNA polymerase (gp5), encoded by gene 5, forms a tight, 11 complex with Escherichia coli thioredoxin. By a mechanism that is not fully understood, thioredoxin acts as a processivity factor and converts gp5 from a distributive polymerase into a highly processive one. We use a single-molecule imaging approach to visualize the interaction of fluorescently labeled T7 DNA polymerase with double-stranded DNA. We have observed T7 gp5, both with and without thioredoxin, binding nonspecifically to double-stranded DNA and diffusing along the duplex. The gp5/thioredoxin complex remains tightly bound to the DNA while diffusing, whereas gp5 without thioredoxin undergoes frequent dissociation from and rebinding to the DNA. These observations suggest that thioredoxin increases the processivity of T7 DNA polymerase by suppressing microscopic hopping on and off the DNA and keeping the complex tightly bound to the duplex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Richardson CC. Bacteriophage T7: Minimal requirements for the replication of a duplex DNA molecule. Cell. 1983;33(2):315–317. - PubMed
-
- Tabor S, Huber H, Richardson C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987;262(33):16212–16223. - PubMed
-
- Modrich P, Richardson CC. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: An enzyme composed of phage- and host-specific subunits. J Biol Chem. 1975;250(14):5515–5522. - PubMed
-
- Huber HE, Tabor S, Richardson CC. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987;262(33):16224–16232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

