Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole
- PMID: 20080683
- PMCID: PMC2836627
- DOI: 10.1073/pnas.0911168107
Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole
Abstract
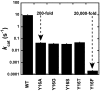
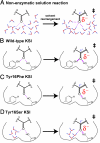
The catalytic importance of enzyme active-site interactions is frequently assessed by mutating specific residues and measuring the resulting rate reductions. This approach has been used in bacterial ketosteroid isomerase to probe the energetic importance of active-site hydrogen bonds donated to the dienolate reaction intermediate. The conservative Tyr16Phe mutation impairs catalysis by 10(5)-fold, far larger than the effects of hydrogen bond mutations in other enzymes. However, the less-conservative Tyr16Ser mutation, which also perturbs the Tyr16 hydrogen bond, results in a less-severe 10(2)-fold rate reduction. To understand the paradoxical effects of these mutations and clarify the energetic importance of the Tyr16 hydrogen bond, we have determined the 1.6-A resolution x-ray structure of the intermediate analogue, equilenin, bound to the Tyr16Ser mutant and measured the rate effects of mutating Tyr16 to Ser, Thr, Ala, and Gly. The nearly identical 200-fold rate reductions of these mutations, together with the 6.4-A distance observed between the Ser16 hydroxyl and equilenin oxygens in the x-ray structure, strongly suggest that the more moderate rate effect of this mutant is not due to maintenance of a hydrogen bond from Ser at position 16. These results, additional spectroscopic observations, and prior structural studies suggest that the Tyr16Phe mutation results in unfavorable interactions with the dienolate intermediate beyond loss of a hydrogen bond, thereby exaggerating the apparent energetic benefit of the Tyr16 hydrogen bond relative to the solution reaction. These results underscore the complex energetics of hydrogen bonding interactions and site-directed mutagenesis experiments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
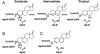


Similar articles
-
Hydrogen bonding in the active site of ketosteroid isomerase: electronic inductive effects and hydrogen bond coupling.Biochemistry. 2010 Dec 7;49(48):10339-48. doi: 10.1021/bi101428e. Epub 2010 Nov 12. Biochemistry. 2010. PMID: 21049962 Free PMC article.
-
Contribution of a low-barrier hydrogen bond to catalysis is not significant in ketosteroid isomerase.Mol Cells. 2015 May;38(5):409-15. doi: 10.14348/molcells.2015.2266. Epub 2015 May 7. Mol Cells. 2015. PMID: 25947291 Free PMC article.
-
Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization.J Biol Chem. 1999 Nov 12;274(46):32863-8. doi: 10.1074/jbc.274.46.32863. J Biol Chem. 1999. PMID: 10551849
-
Structure and enzymology of Delta5-3-ketosteroid isomerase.Curr Opin Struct Biol. 2001 Dec;11(6):674-8. doi: 10.1016/s0959-440x(01)00268-8. Curr Opin Struct Biol. 2001. PMID: 11751047 Review.
-
Mechanistic insights from the three-dimensional structure of 3-oxo-Delta(5)-steroid isomerase.Arch Biochem Biophys. 1999 Oct 1;370(1):9-15. doi: 10.1006/abbi.1999.1384. Arch Biochem Biophys. 1999. PMID: 10496971 Review.
Cited by
-
Intrigues and intricacies of the biosynthetic pathways for the enzymatic quinocofactors: PQQ, TTQ, CTQ, TPQ, and LTQ.Chem Rev. 2014 Apr 23;114(8):4343-65. doi: 10.1021/cr400475g. Epub 2013 Dec 18. Chem Rev. 2014. PMID: 24350630 Free PMC article. Review. No abstract available.
-
Systematic investigation of the link between enzyme catalysis and cold adaptation.Elife. 2022 Jan 12;11:e72884. doi: 10.7554/eLife.72884. Elife. 2022. PMID: 35019838 Free PMC article.
-
Mutation at a strictly conserved, active site tyrosine in the copper amine oxidase leads to uncontrolled oxygenase activity.Biochemistry. 2010 Aug 31;49(34):7393-402. doi: 10.1021/bi100643y. Biochemistry. 2010. PMID: 20684524 Free PMC article.
-
Ensemble-function relationships to dissect mechanisms of enzyme catalysis.Sci Adv. 2022 Oct 14;8(41):eabn7738. doi: 10.1126/sciadv.abn7738. Epub 2022 Oct 14. Sci Adv. 2022. PMID: 36240280 Free PMC article.
-
Specificity in transition state binding: the Pauling model revisited.Biochemistry. 2013 Mar 26;52(12):2021-35. doi: 10.1021/bi301491r. Epub 2013 Feb 4. Biochemistry. 2013. PMID: 23327224 Free PMC article. Review.
References
-
- Fersht AR. Structure and Mechanism in Protein Science. New York: W.H. Freeman and Company; 1999.
-
- Radzicka A, Wolfenden R. A proficient enzyme. Science. 1995;267:90–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

