Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment
- PMID: 20080688
- PMCID: PMC2824379
- DOI: 10.1073/pnas.0908682107
Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment
Abstract
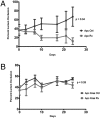
Most genetically engineered mouse (GEM) models for colon cancer are based on tissuewide or germline gene modification, resulting in tumors predominantly of the small intestine. Several of these models involve modification of the adenomatous polyposis coli (Apc) gene and are excellent models for familial cancer predisposition syndromes. We have developed a stochastic somatic mutation model for sporadic colon cancer that presents with isolated primary tumors in the distal colon and recapitulates the entire adenoma-carcinoma-metastasis axis seen in human colon cancer. Using this model, we have analyzed tumors that are either solely mutant in the Apc gene or in combination with another colon cancer-associated mutant gene, the Kras G12D allele. Because of the restricted location in the distal colon, the natural history of the tumors can be analyzed by serial colonoscopy. As the mammalian target of rapamycin (mTOR) pathway is a critical component of the complex signaling network in colon cancer, we used this model to assess the efficacy of mTOR blockade through rapamycin treatment of mice with established tumors. After treatment, Apc mutant tumors were more than 80% smaller than control tumors. However, tumors that possessed both Apc and Kras mutations did not respond to rapamycin treatment. These studies suggest that mTOR inhibitors should be further explored as potential colorectal cancer therapies in patients whose tumors do not have activating mutations in KRAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. - PubMed
-
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. - PubMed
-
- Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. - PubMed
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

