Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver
- PMID: 20080689
- PMCID: PMC2824398
- DOI: 10.1073/pnas.0911427107
Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver
Abstract
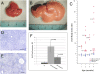
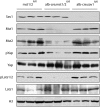
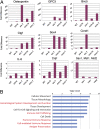
How organ size is controlled in mammals is not currently understood. In Drosophila the Hippo signaling pathway functions to suppress growth in imaginal discs and has been suggested to control organ size. To investigate the role of hippo signaling in regulation of mammalian organ size we have generated conditional alleles of Sav1, mst1, and mst2, orthologs of Drosophila Salvador and hippo, respectively. Specific deletion of both mst1 and mst2 in hepatocytes results in significantly enlarged livers due to excessive proliferation. By the age of 5-6 months, mst1/2 conditional mutant livers have multiple foci of liver tumors, indicating that the combined activities of mst1 and mst2 act as redundant tumor suppressors in hepatocytes. Similar findings were obtained with liver-specific deletion of Sav1, a second core Hippo signaling component that facilitates activation of mst1 and mst2. Tumors from sav1 mutants exhibited varied morphology, suggesting a mixed-lineage origin of tumor-initiating cells. Transcriptional profiling of liver tissues from both mst1/2 and sav1 conditional mutants revealed a network of Hippo signaling regulated genes with specific enrichment for genes involved in immune and inflammatory responses. Histological and immunological characterization of mst1/2 double mutant liver tissues revealed abundant accumulation of adult facultative stem cells termed oval cells in periductal regions. Because oval cells induction is commonly associated with liver injury and tumor formation, it is likely that these cells contribute to the enlarged livers and hepatomas that we observe in sav1 and mst1/2 mutants. Taken together, our results demonstrate that the Hippo signaling pathway is a critical regulator of mammalian liver growth and a potent suppressor of liver tumor formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. - PubMed
-
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. - PubMed
-
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. - PubMed
-
- St John MA, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

