Protein phosphatase PHLPP1 controls the light-induced resetting of the circadian clock
- PMID: 20080691
- PMCID: PMC2824391
- DOI: 10.1073/pnas.0910292107
Protein phosphatase PHLPP1 controls the light-induced resetting of the circadian clock
Abstract
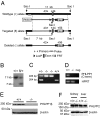
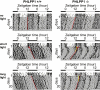
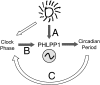
The pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) differentially attenuates Akt, PKC, and ERK1/2 signaling, thereby controlling the duration and amplitude of responses evoked by these kinases. PHLPP1 is expressed in the mammalian central clock, the suprachiasmatic nucleus, where it oscillates in a circadian fashion. To explore the role of PHLPP1 in vivo, we have generated mice with a targeted deletion of the PHLPP1 gene. Here we show that PHLPP1-null mice, although displaying normal circadian rhythmicity, have a drastically impaired capacity to stabilize the circadian period after light-induced resetting, producing a large phase shift after light resetting. Our findings reveal that PHLPP1 exerts a previously unappreciated role in circadian control, governing the consolidation of circadian periodicity after resetting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mislocalization of the E3 ligase, β-transducin repeat-containing protein 1 (β-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt.J Biol Chem. 2011 Jun 3;286(22):19777-88. doi: 10.1074/jbc.M111.237081. Epub 2011 Mar 28. J Biol Chem. 2011. PMID: 21454620 Free PMC article.
-
Deletion of the PH-domain and Leucine-rich Repeat Protein Phosphatase 1 (Phlpp1) Increases Fibroblast Growth Factor (Fgf) 18 Expression and Promotes Chondrocyte Proliferation.J Biol Chem. 2015 Jun 26;290(26):16272-80. doi: 10.1074/jbc.M114.612937. Epub 2015 May 7. J Biol Chem. 2015. PMID: 25953896 Free PMC article.
-
Phlpp1 alters the murine chondrocyte phospho-proteome during endochondral bone formation.Bone. 2024 Dec;189:117265. doi: 10.1016/j.bone.2024.117265. Epub 2024 Sep 29. Bone. 2024. PMID: 39349089
-
PHLiPPing the switch on Akt and protein kinase C signaling.Trends Endocrinol Metab. 2008 Aug;19(6):223-30. doi: 10.1016/j.tem.2008.04.001. Epub 2008 May 27. Trends Endocrinol Metab. 2008. PMID: 18511290 Free PMC article. Review.
-
Evidence for Internal Desynchrony Caused by Circadian Clock Resetting.Yale J Biol Med. 2019 Jun 27;92(2):259-270. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249487 Free PMC article. Review.
Cited by
-
Histone deacetylase 3 suppression increases PH domain and leucine-rich repeat phosphatase (Phlpp)1 expression in chondrocytes to suppress Akt signaling and matrix secretion.J Biol Chem. 2013 Apr 5;288(14):9572-9582. doi: 10.1074/jbc.M112.423723. Epub 2013 Feb 13. J Biol Chem. 2013. PMID: 23408427 Free PMC article.
-
Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP): a new player in cell signaling.J Biol Chem. 2012 Feb 3;287(6):3610-6. doi: 10.1074/jbc.R111.318675. Epub 2011 Dec 5. J Biol Chem. 2012. PMID: 22144674 Free PMC article.
-
Pleckstrin Homology (PH) Domain Leucine-rich Repeat Protein Phosphatase Controls Cell Polarity by Negatively Regulating the Activity of Atypical Protein Kinase C.J Biol Chem. 2016 Nov 25;291(48):25167-25178. doi: 10.1074/jbc.M116.740639. Epub 2016 Oct 19. J Biol Chem. 2016. PMID: 27760826 Free PMC article.
-
PHLPP-mediated dephosphorylation of S6K1 inhibits protein translation and cell growth.Mol Cell Biol. 2011 Dec;31(24):4917-27. doi: 10.1128/MCB.05799-11. Epub 2011 Oct 10. Mol Cell Biol. 2011. PMID: 21986499 Free PMC article.
-
Inhibition of Phlpp1 preserves the mechanical integrity of articular cartilage in a murine model of post-traumatic osteoarthritis.Osteoarthritis Cartilage. 2024 Jun;32(6):680-689. doi: 10.1016/j.joca.2024.01.008. Epub 2024 Mar 1. Osteoarthritis Cartilage. 2024. PMID: 38432607 Free PMC article.
References
-
- Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

