The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function
- PMID: 20080692
- PMCID: PMC2824380
- DOI: 10.1073/pnas.0908735107
The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function
Abstract
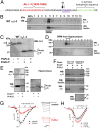
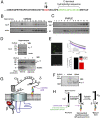
Voltage-gated calcium channels are thought to exist in the plasma membrane as heteromeric proteins, in which the alpha1 subunit is associated with two auxiliary subunits, the intracellular beta subunit and the alpha(2)delta subunit; both of these subunits influence the trafficking and properties of Ca(V)1 and Ca(V)2 channels. The alpha(2)delta subunits have been described as type I transmembrane proteins, because they have an N-terminal signal peptide and a C-terminal hydrophobic and potentially transmembrane region. However, because they have very short C-terminal cytoplasmic domains, we hypothesized that the alpha(2)delta proteins might be associated with the plasma membrane through a glycosylphosphatidylinositol (GPI) anchor attached to delta rather than a transmembrane domain. Here, we provide biochemical, immunocytochemical, and mutational evidence to show that all of the alpha(2)delta subunits studied, alpha(2)delta-1, alpha(2)delta-2, and alpha(2)delta-3, show all of the properties expected of GPI-anchored proteins, both when heterologously expressed and in native tissues. They are substrates for prokaryotic phosphatidylinositol-phospholipase C (PI-PLC) and trypanosomal GPI-PLC, which release the alpha(2)delta proteins from membranes and intact cells and expose a cross-reacting determinant epitope. PI-PLC does not affect control transmembrane or membrane-associated proteins. Furthermore, mutation of the predicted GPI-anchor sites markedly reduced plasma membrane and detergent-resistant membrane localization of alpha(2)delta subunits. We also show that GPI anchoring of alpha(2)delta subunits is necessary for their function to enhance calcium currents, and PI-PLC treatment only reduces calcium current density when alpha(2)delta subunits are coexpressed. In conclusion, this study redefines our understanding of alpha(2)delta subunits, both in terms of their role in calcium-channel function and other roles in synaptogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Flockerzi V, et al. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323:66–68. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Ellis SB, et al. Sequence and expression of mRNAs encoding the α 1 and α 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. - PubMed
-
- De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. α 2 and δ are encoded by the same gene. J Biol Chem. 1990;265:14738–14741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

