NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis
- PMID: 20080708
- PMCID: PMC2824315
- DOI: 10.1073/pnas.0909015107
NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis
Abstract
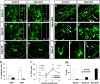
After their generation and specification in periventricular regions, neuronal precursors maintain an immature and migratory state until their arrival in the respective target structures. Only here are terminal differentiation and synaptic integration induced. Although the molecular control of neuronal specification has started to be elucidated, little is known about the factors that control the latest maturation steps. We aimed at identifying factors that induce terminal differentiation during postnatal and adult neurogenesis, thereby focusing on the generation of periglomerular interneurons in the olfactory bulb. We isolated neuronal precursors and mature neurons from the periglomerular neuron lineage and analyzed their gene expression by microarray. We found that expression of the bHLH transcription factor NeuroD1 strikingly coincides with terminal differentiation. Using brain electroporation, we show that overexpression of NeuroD1 in the periventricular region in vivo leads to the rapid appearance of cells with morphological and molecular characteristics of mature neurons in the subventricular zone and rostral migratory stream. Conversely, shRNA-induced knockdown of NeuroD1 inhibits terminal neuronal differentiation. Thus, expression of a single transcription factor is sufficient to induce neuronal differentiation of neural progenitors in regions that normally do not show addition of new neurons. These results suggest a considerable potential of NeuroD1 for use in cell-therapeutic approaches in the nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

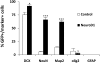

References
-
- Edlund T, Jessell TM. Progression from extrinsic to intrinsic signaling in cell fate specification: A view from the nervous system. Cell. 1999;96:211–224. - PubMed
-
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. - PubMed
-
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

