How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA
- PMID: 20080730
- PMCID: PMC2796979
- DOI: 10.1073/pnas.0911099107
How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA
Abstract
The POT1-TPP1 heterodimer, the major telomere-specific single-stranded DNA-binding protein in mammalian cells, protects chromosome ends and contributes to the regulation of telomerase. The recent discovery of telomeric RNA raises the question of how POT1 faithfully binds telomeric ssDNA and avoids illicit RNA binding that could result in its depletion from telomeres. Here we show through binding studies that a single deoxythymidine in a telomeric repeat dictates the DNA versus RNA discrimination by human POT1 and mouse POT1A. We solve the crystal structure of hPOT1 bound to DNA with a ribouridine in lieu of the critical deoxythymidine and show that this substitution results in burying the 2(')-hydroxyl group in a hydrophobic region (Phe62) of POT1 in addition to eliminating favorable hydrogen-bonding interactions at the POT1-nucleic acid interface. At amino acid 62, Phe discriminates against RNA binding and Tyr allows RNA binding. We further show that TPP1 greatly augments POT1's discrimination against RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
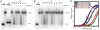
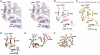
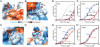
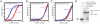
Similar articles
-
TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase.Nature. 2007 Feb 1;445(7127):559-62. doi: 10.1038/nature05469. Epub 2007 Jan 21. Nature. 2007. PMID: 17237767
-
DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA.Nature. 2003 Nov 13;426(6963):198-203. doi: 10.1038/nature02092. Nature. 2003. PMID: 14614509
-
Coordinated interactions of multiple POT1-TPP1 proteins with telomere DNA.J Biol Chem. 2013 Jun 7;288(23):16361-16370. doi: 10.1074/jbc.M113.471896. Epub 2013 Apr 24. J Biol Chem. 2013. PMID: 23616058 Free PMC article.
-
Structural biology of telomeres and telomerase.Cell Mol Life Sci. 2020 Jan;77(1):61-79. doi: 10.1007/s00018-019-03369-x. Epub 2019 Nov 14. Cell Mol Life Sci. 2020. PMID: 31728577 Free PMC article. Review.
-
Shelterin: the protein complex that shapes and safeguards human telomeres.Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
Cited by
-
Role of POT1 in Human Cancer.Cancers (Basel). 2020 Sep 24;12(10):2739. doi: 10.3390/cancers12102739. Cancers (Basel). 2020. PMID: 32987645 Free PMC article. Review.
-
OB-fold Families of Genome Guardians: A Universal Theme Constructed From the Small β-barrel Building Block.Front Mol Biosci. 2022 Feb 11;9:784451. doi: 10.3389/fmolb.2022.784451. eCollection 2022. Front Mol Biosci. 2022. PMID: 35223988 Free PMC article. Review.
-
Multiple facets of TPP1 in telomere maintenance.Biochim Biophys Acta. 2014 Sep;1844(9):1550-9. doi: 10.1016/j.bbapap.2014.04.014. Epub 2014 Apr 26. Biochim Biophys Acta. 2014. PMID: 24780581 Free PMC article. Review.
-
How DNA damage and non-canonical nucleotides alter the telomerase catalytic cycle.DNA Repair (Amst). 2021 Nov;107:103198. doi: 10.1016/j.dnarep.2021.103198. Epub 2021 Jul 31. DNA Repair (Amst). 2021. PMID: 34371388 Free PMC article. Review.
-
POT1 loss-of-function variants predispose to familial melanoma.Nat Genet. 2014 May;46(5):478-481. doi: 10.1038/ng.2947. Epub 2014 Mar 30. Nat Genet. 2014. PMID: 24686849 Free PMC article.
References
-
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. - PubMed
-
- Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. - PubMed
-
- de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

