A structure-based mechanism for benzalacetone synthase from Rheum palmatum
- PMID: 20080733
- PMCID: PMC2818918
- DOI: 10.1073/pnas.0909982107
A structure-based mechanism for benzalacetone synthase from Rheum palmatum
Abstract
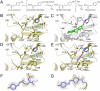
Benzalacetone synthase (BAS), a plant-specific type III polyketide synthase (PKS), catalyzes a one-step decarboxylative condensation of malonyl-CoA and 4-coumaroyl-CoA to produce the diketide benzalacetone. We solved the crystal structures of both the wild-type and chalcone-producing I207L/L208F mutant of Rheum palmatum BAS at 1.8 A resolution. In addition, we solved the crystal structure of the wild-type enzyme, in which a monoketide coumarate intermediate is covalently bound to the catalytic cysteine residue, at 1.6 A resolution. This is the first direct evidence that type III PKS utilizes the cysteine as the nucleophile and as the attachment site for the polyketide intermediate. The crystal structures revealed that BAS utilizes an alternative, novel active-site pocket for locking the aromatic moiety of the coumarate, instead of the chalcone synthase's coumaroyl-binding pocket, which is lost in the active-site of the wild-type enzyme and restored in the I207L/L208F mutant. Furthermore, the crystal structures indicated the presence of a putative nucleophilic water molecule which forms hydrogen bond networks with the Cys-His-Asn catalytic triad. This suggested that BAS employs novel catalytic machinery for the thioester bond cleavage of the enzyme-bound diketide intermediate and the final decarboxylation reaction to produce benzalacetone. These findings provided a structural basis for the functional diversity of the type III PKS enzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
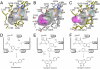

Similar articles
-
How structural subtleties lead to molecular diversity for the type III polyketide synthases.J Biol Chem. 2019 Oct 11;294(41):15121-15136. doi: 10.1074/jbc.REV119.006129. Epub 2019 Aug 30. J Biol Chem. 2019. PMID: 31471316 Free PMC article. Review.
-
Benzalacetone synthase.Front Plant Sci. 2012 Mar 21;3:57. doi: 10.3389/fpls.2012.00057. eCollection 2012. Front Plant Sci. 2012. PMID: 22645592 Free PMC article.
-
Structure function analysis of benzalacetone synthase from Rheum palmatum.Bioorg Med Chem Lett. 2007 Jun 1;17(11):3161-6. doi: 10.1016/j.bmcl.2007.03.029. Epub 2007 Mar 15. Bioorg Med Chem Lett. 2007. PMID: 17383877
-
Structure-based engineering of benzalacetone synthase.Bioorg Med Chem Lett. 2010 Sep 1;20(17):5099-103. doi: 10.1016/j.bmcl.2010.07.022. Epub 2010 Jul 11. Bioorg Med Chem Lett. 2010. PMID: 20667730
-
Engineering of plant polyketide biosynthesis.Chem Pharm Bull (Tokyo). 2008 Nov;56(11):1505-14. doi: 10.1248/cpb.56.1505. Chem Pharm Bull (Tokyo). 2008. PMID: 18981598 Review.
Cited by
-
How structural subtleties lead to molecular diversity for the type III polyketide synthases.J Biol Chem. 2019 Oct 11;294(41):15121-15136. doi: 10.1074/jbc.REV119.006129. Epub 2019 Aug 30. J Biol Chem. 2019. PMID: 31471316 Free PMC article. Review.
-
Structural basis for the one-pot formation of the diarylheptanoid scaffold by curcuminoid synthase from Oryza sativa.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19778-83. doi: 10.1073/pnas.1011499107. Epub 2010 Nov 1. Proc Natl Acad Sci U S A. 2010. PMID: 21041675 Free PMC article.
-
An Overview of the Medicinally Important Plant Type III PKS Derived Polyketides.Front Plant Sci. 2021 Oct 14;12:746908. doi: 10.3389/fpls.2021.746908. eCollection 2021. Front Plant Sci. 2021. PMID: 34721474 Free PMC article. Review.
-
Molecular architectures of benzoic acid-specific type III polyketide synthases.Acta Crystallogr D Struct Biol. 2017 Dec 1;73(Pt 12):1007-1019. doi: 10.1107/S2059798317016618. Epub 2017 Nov 30. Acta Crystallogr D Struct Biol. 2017. PMID: 29199980 Free PMC article.
-
Benzalacetone synthase.Front Plant Sci. 2012 Mar 21;3:57. doi: 10.3389/fpls.2012.00057. eCollection 2012. Front Plant Sci. 2012. PMID: 22645592 Free PMC article.
References
-
- Schröder J. Comprehensive Natural Products Chemistry. Vol 1. Oxford: Elsevier; 1999. pp. 749–771.
-
- Austin MB, Noel JP. The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep. 2003;20:79–110. - PubMed
-
- Morita H, Abe I, Noguchi H. Comprehensive Natural Products Chemistry. Oxford, in press: Elsevier; 2009.
-
- Abe I, Takahashi Y, Morita H, Noguchi H. Benzalacetone synthase. A novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum. Eur J Biochem. 2001;268:3354–3359. - PubMed
-
- Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol. 1999;6:775–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

