Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice
- PMID: 20080762
- PMCID: PMC2818887
- DOI: 10.1073/pnas.0912134107
Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice
Abstract
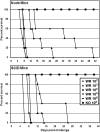
The threat of smallpox as a bioweapon and the emerging threat of human monkeypox, among other poxviral diseases, highlight the need for effective poxvirus countermeasures. ST-246, which targets the F13L protein in vaccinia virus and its homologs in other orthopoxvirus species, provides full protection from lethal poxviral disease in numerous animal models and seems to be safe in humans. All previous evaluations of ST-246 efficacy have been in immunocompetent animals. However, the risk of severe poxviral disease is greater in immunodeficient hosts. Here we report on the efficacy of ST-246 in preventing or treating lethal poxviral disease in immunodeficient mice. After lethal challenge with the Western Reserve strain of vaccinia, Nude, SCID, and J(H) knockout mice additionally depleted of CD4(+) and CD8(+) T cells were not fully protected by ST-246, although survival was significantly extended. However, CD4(+) T cell deficient, CD8(+) T cell deficient, J(H) knockout, and J(H) knockout mice also deficient for CD4(+) or CD8(+) T cells survived lethal challenge when treated with ST-246 starting on the day of challenge. Delaying treatment until 72 h after infection reduced ST-246 efficacy in some models but provided full protection from lethal challenge in most. These findings suggest that ST-246 may be effective in controlling smallpox or other pathogenic orthopoxviruses in some immunodeficient human populations for whom the vaccine is contraindicated.
Conflict of interest statement
Conflict of interest statement: All authors are employed by SIGA Technologies.
Figures


Similar articles
-
An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge.J Virol. 2005 Oct;79(20):13139-49. doi: 10.1128/JVI.79.20.13139-13149.2005. J Virol. 2005. PMID: 16189015 Free PMC article.
-
ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation.Antimicrob Agents Chemother. 2009 Dec;53(12):4999-5009. doi: 10.1128/AAC.00678-09. Epub 2009 Sep 14. Antimicrob Agents Chemother. 2009. PMID: 19752270 Free PMC article.
-
Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (Smallpox).Antimicrob Agents Chemother. 2013 Dec;57(12):6246-53. doi: 10.1128/AAC.00977-13. Epub 2013 Oct 7. Antimicrob Agents Chemother. 2013. PMID: 24100494 Free PMC article.
-
Tecovirimat: A journey from discovery to mechanistic insights in poxvirus inhibition.PLoS Pathog. 2025 May 16;21(5):e1013140. doi: 10.1371/journal.ppat.1013140. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40378361 Free PMC article. Review.
-
An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications.Expert Rev Anti Infect Ther. 2021 Mar;19(3):331-344. doi: 10.1080/14787210.2020.1819791. Epub 2020 Sep 15. Expert Rev Anti Infect Ther. 2021. PMID: 32882158 Free PMC article. Review.
Cited by
-
A review of Mpox: Biological characteristics, epidemiology, clinical features, diagnosis, treatment, and prevention strategies.Exploration (Beijing). 2024 Oct 8;5(2):20230112. doi: 10.1002/EXP.20230112. eCollection 2025 Apr. Exploration (Beijing). 2024. PMID: 40395760 Free PMC article. Review.
-
Monkeypox Virus Infections in Humans.Clin Microbiol Rev. 2022 Dec 21;35(4):e0009222. doi: 10.1128/cmr.00092-22. Epub 2022 Nov 14. Clin Microbiol Rev. 2022. PMID: 36374082 Free PMC article. Review.
-
A human recombinant analogue to plasma-derived vaccinia immunoglobulin prophylactically and therapeutically protects against lethal orthopoxvirus challenge.Antiviral Res. 2021 Nov;195:105179. doi: 10.1016/j.antiviral.2021.105179. Epub 2021 Sep 13. Antiviral Res. 2021. PMID: 34530009 Free PMC article.
-
Potential therapeutic targets for Mpox: the evidence to date.Expert Opin Ther Targets. 2023 Jan-Jun;27(6):419-431. doi: 10.1080/14728222.2023.2230361. Epub 2023 Jul 4. Expert Opin Ther Targets. 2023. PMID: 37368464 Free PMC article.
-
Poxvirus interleukin-4 expression overcomes inherent resistance and vaccine-induced immunity: pathogenesis, prophylaxis, and antiviral therapy.Virology. 2011 Jan 20;409(2):328-37. doi: 10.1016/j.virol.2010.10.021. Epub 2010 Nov 10. Virology. 2011. PMID: 21071055 Free PMC article.
References
-
- Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1279–1282. - PubMed
-
- Henderson DA, et al. Working Group on Civilian Biodefense. Smallpox as a biological weapon: Medical and public health management. JAMA. 1999;281:2127–2137. - PubMed
-
- Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007;2:17–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

