VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy
- PMID: 20080765
- PMCID: PMC2818941
- DOI: 10.1073/pnas.0911661107
VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy
Abstract
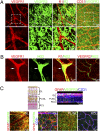
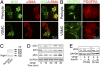
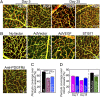
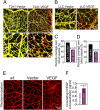
VEGF coordinates complex regulation of cellular regeneration and interactions between endothelial and perivascular cells; dysfunction of the VEGF signaling system leads to retinopathy. Here, we show that systemic delivery of VEGF and placental growth factor (PlGF) by protein implantation, tumors, and adenoviral vectors ablates pericytes from the mature retinal vasculature through the VEGF receptor 1 (VEGFR1)-mediated signaling pathway, leading to increased vascular leakage. In contrast, we demonstrate VEGF receptor 2 (VEGFR2) is primarily expressed in nonvascular photoreceptors and ganglion cells. Moreover, blockade of VEGFR1 but not VEGFR2 significantly restores pericyte saturation in mature retinal vessels. Our findings link VEGF and PlGF to cancer-associated retinopathy, reveal the molecular mechanisms of VEGFR1 ligand-mediated retinopathy, and define VEGFR1 as an important target of antiangiogenic therapy for treatment of retinopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. - PubMed
-
- Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. - PubMed
-
- Buzney SM, Frank RN, Robison WG., Jr Retinal capillaries: Proliferation of mural cells in vitro. Science. 1975;190:985–986. - PubMed
-
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

