Do mycobacteria produce endospores?
- PMID: 20080769
- PMCID: PMC2818926
- DOI: 10.1073/pnas.0911299107
Do mycobacteria produce endospores?
Abstract
The genus Mycobacterium, which is a member of the high G+C group of Gram-positive bacteria, includes important pathogens, such as M. tuberculosis and M. leprae. A recent publication in PNAS reported that M. marinum and M. bovis bacillus Calmette-Guérin produce a type of spore known as an endospore, which had been observed only in the low G+C group of Gram-positive bacteria. Evidence was presented that the spores were similar to endospores in ultrastructure, in heat resistance and in the presence of dipicolinic acid. Here, we report that the genomes of Mycobacterium species and those of other high G+C Gram-positive bacteria lack orthologs of many, if not all, highly conserved genes diagnostic of endospore formation in the genomes of low G+C Gram-positive bacteria. We also failed to detect the presence of endospores by light microscopy or by testing for heat-resistant colony-forming units in aged cultures of M. marinum. Finally, we failed to recover heat-resistant colony-forming units from frogs chronically infected with M. marinum. We conclude that it is unlikely that Mycobacterium is capable of endospore formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

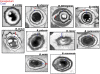

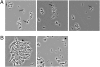

Similar articles
-
Sporulation in mycobacteria.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10781-6. doi: 10.1073/pnas.0904104106. Epub 2009 Jun 16. Proc Natl Acad Sci U S A. 2009. PMID: 19541637 Free PMC article.
-
The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria.Genome Biol. 2001;2(10):RESEARCH0044. doi: 10.1186/gb-2001-2-10-research0044. Epub 2001 Sep 19. Genome Biol. 2001. PMID: 11597336 Free PMC article.
-
The Impact of Genome Region of Difference 4 (RD4) on Mycobacterial Virulence and BCG Efficacy.Front Cell Infect Microbiol. 2017 Jun 8;7:239. doi: 10.3389/fcimb.2017.00239. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28642843 Free PMC article.
-
The secret lives of the pathogenic mycobacteria.Annu Rev Microbiol. 2003;57:641-76. doi: 10.1146/annurev.micro.57.030502.091033. Annu Rev Microbiol. 2003. PMID: 14527294 Review.
-
Mycobacterium tuberculosis pathogenicity viewed through the lens of molecular Koch's postulates.Curr Opin Microbiol. 2020 Apr;54:103-110. doi: 10.1016/j.mib.2020.01.011. Epub 2020 Feb 13. Curr Opin Microbiol. 2020. PMID: 32062573 Review.
Cited by
-
Single-cell elemental analysis of bacteria: quantitative analysis of polyphosphates in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2012 May 24;2:63. doi: 10.3389/fcimb.2012.00063. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919654 Free PMC article.
-
Conservation and Evolution of the Sporulation Gene Set in Diverse Members of the Firmicutes.J Bacteriol. 2022 Jun 21;204(6):e0007922. doi: 10.1128/jb.00079-22. Epub 2022 May 31. J Bacteriol. 2022. PMID: 35638784 Free PMC article.
-
Assembly of the BclB glycoprotein into the exosporium and evidence for its role in the formation of the exosporium 'cap' structure in Bacillus anthracis.Mol Microbiol. 2012 Dec;86(5):1073-84. doi: 10.1111/mmi.12042. Epub 2012 Oct 11. Mol Microbiol. 2012. PMID: 22989026 Free PMC article.
-
Initiation of Post-Primary Tuberculosis of the Lungs: Exploring the Secret Role of Bone Marrow Derived Stem Cells.Front Immunol. 2021 Jan 21;11:594572. doi: 10.3389/fimmu.2020.594572. eCollection 2020. Front Immunol. 2021. PMID: 33584661 Free PMC article. Review.
-
Recent progress in Bacillus subtilis sporulation.FEMS Microbiol Rev. 2012 Jan;36(1):131-48. doi: 10.1111/j.1574-6976.2011.00310.x. Epub 2011 Oct 25. FEMS Microbiol Rev. 2012. PMID: 22091839 Free PMC article. Review.
References
-
- Kochi A. Global challenge of tuberculosis. Lancet. 1994;344:608–609. - PubMed
-
- Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. - PubMed
-
- Driks A. Maximum shields: The assembly and function of the bacterial spore coat. Trends Microbiol. 2002;10:251–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

