Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches
- PMID: 20080785
- PMCID: PMC2796905
- DOI: 10.1073/pnas.0902639106
Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches
Abstract
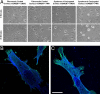
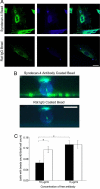
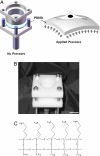
The ability of cells to respond to external mechanical stimulation is a complex and robust process involving a diversity of molecular interactions. Although mechanotransduction has been heavily studied, many questions remain regarding the link between physical stimulation and biochemical response. Of significant interest has been the contribution of the transmembrane proteins involved, and integrins in particular, because of their connectivity to both the extracellular matrix and the cytoskeleton. Here, we demonstrate the existence of a mechanically based initiation molecule, syndecan-4. We first demonstrate the ability of syndecan-4 molecules to support cell attachment and spreading without the direct extracellular binding of integrins. We also examine the distribution of focal adhesion-associated proteins through controlling surface interactions of beads with molecular specificity in binding to living cells. Furthermore, after adhering cells to elastomeric membranes via syndecan-4-specific attachments we mechanically strained the cells via our mechanical stimulation and polymer surface chemical modification approach. We found ERK phosphorylation similar to that shown for mechanotransductive response for integrin-based cell attachments through our elastomeric membrane-based approach and optical magnetic twisting cytometry for syndecan-4. Finally, through the use of cytoskeletal disruption agents, this mechanical signaling was shown to be actin cytoskeleton dependent. We believe that these results will be of interest to a wide range of fields, including mechanotransduction, syndecan biology, and cell-material interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
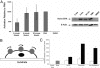
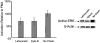
References
-
- Wang Y, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. - PubMed
-
- Engler A, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Ferrer I, et al. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and dementia with Lewy bodies. J Neural Transm. 2001;108:1383–1396. - PubMed
-
- Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–25280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

