Muscarinic receptor stimulation of D-aspartate uptake into human SH-SY5Y neuroblastoma cells is attenuated by hypoosmolarity
- PMID: 20080957
- PMCID: PMC2846019
- DOI: 10.1124/jpet.109.164277
Muscarinic receptor stimulation of D-aspartate uptake into human SH-SY5Y neuroblastoma cells is attenuated by hypoosmolarity
Abstract
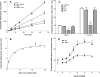
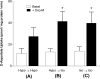
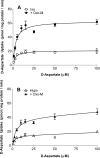
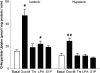
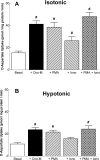
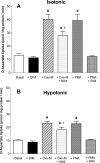
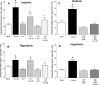
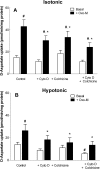
In addition to its function as an excitatory neurotransmitter, glutamate plays a major role as an osmolyte within the central nervous system (CNS). Accordingly, mechanisms that regulate glutamate release and uptake are of physiological importance not only during conditions in which cell volume remains constant but also when cells are subjected to hypoosmotic stress. In the present study, the ability of muscarinic cholinergic receptors (mAChRs) to regulate the uptake of glutamate (monitored as D-aspartate) into human SH-SY5Y neuroblastoma cells under isotonic or hypotonic conditions has been examined. In isotonic media, agonist activation of mAChRs resulted in a significant increase (250-300% of control) in the uptake of D-aspartate and, concurrently, a cellular redistribution of the excitatory amino acid transporter 3 (EAAT3) to the plasma membrane. mAChR-mediated increases in d-aspartate uptake were potently blocked by the EAAT3 inhibitor l-beta-threo-benzyl-aspartate. In hypotonic media, the ability of mAChR activation to facilitate D-aspartate uptake was significantly attenuated (40-50%), and the cellular distribution of EAAT3 was disrupted. Reduction of mAChR-stimulated D-aspartate uptake under hypoosmotic conditions could be fully reversed upon re-exposure of the cells to isotonic media. Under both isotonic and hypotonic conditions, mAChR-mediated increases in D-aspartate uptake depended on cytoskeletal integrity, protein kinase C and phosphatidylinositol 3-kinase activities, and the availability of intracellular Ca2+. In contrast, dependence on extracellular Ca2+ was observed only under isotonic conditions. The results suggest that, although the uptake of D-aspartate into SH-SY5Y cells is enhanced after mAChR activation, this process is markedly attenuated by hypoosmolarity.
Figures



Similar articles
-
Muscarinic receptor regulation of osmosensitive taurine transport in human SH-SY5Y neuroblastoma cells.J Neurochem. 2009 Jan;108(2):437-49. doi: 10.1111/j.1471-4159.2008.05773.x. Epub 2008 Oct 8. J Neurochem. 2009. PMID: 19012745 Free PMC article.
-
Potentiation of the osmosensitive release of taurine and D-aspartate from SH-SY5Y neuroblastoma cells after activation of M3 muscarinic cholinergic receptors.J Pharmacol Exp Ther. 2004 Dec;311(3):1097-104. doi: 10.1124/jpet.104.072553. Epub 2004 Aug 3. J Pharmacol Exp Ther. 2004. PMID: 15292461
-
Activation of muscarinic cholinergic receptors on human SH-SY5Y neuroblastoma cells enhances both the influx and efflux of K+ under conditions of hypo-osmolarity.J Pharmacol Exp Ther. 2008 May;325(2):457-65. doi: 10.1124/jpet.107.135475. Epub 2008 Feb 15. J Pharmacol Exp Ther. 2008. PMID: 18281593
-
Activation of muscarinic cholinergic receptors enhances the volume-sensitive efflux of myo-inositol from SH-SY5Y neuroblastoma cells.J Neurochem. 2003 Oct;87(2):476-86. doi: 10.1046/j.1471-4159.2003.02021.x. J Neurochem. 2003. PMID: 14511125
-
Carbachol-stimulated calcium entry in SH-SY5Y human neuroblastoma cells: which route?J Physiol Paris. 1992;86(1-3):77-82. doi: 10.1016/s0928-4257(05)80010-5. J Physiol Paris. 1992. PMID: 1343598 Review.
Cited by
-
Rapid Regulation of Glutamate Transport: Where Do We Go from Here?Neurochem Res. 2022 Jan;47(1):61-84. doi: 10.1007/s11064-021-03329-7. Epub 2021 Apr 24. Neurochem Res. 2022. PMID: 33893911 Free PMC article. Review.
-
Receptor regulation of osmolyte homeostasis in neural cells.J Physiol. 2010 Sep 15;588(Pt 18):3355-64. doi: 10.1113/jphysiol.2010.190777. Epub 2010 May 24. J Physiol. 2010. PMID: 20498228 Free PMC article. Review.
-
The importance of the excitatory amino acid transporter 3 (EAAT3).Neurochem Int. 2016 Sep;98:4-18. doi: 10.1016/j.neuint.2016.05.007. Epub 2016 May 24. Neurochem Int. 2016. PMID: 27233497 Free PMC article. Review.
-
d-Amino Acid Peptide Residualizing Agents for Protein Radioiodination: Effect of Aspartate for Glutamate Substitution.Molecules. 2018 May 20;23(5):1223. doi: 10.3390/molecules23051223. Molecules. 2018. PMID: 29783774 Free PMC article.
References
-
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. (2006) Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1-deficient mouse. Nat Neurosci 9:119–126 - PubMed
-
- Bhardwaj A. (2006) Neurological impact of vasopressin dysregulation and hyponatremia. Ann Neurol 59:229–236 - PubMed
-
- Bianchi MG, Rotoli BM, Dall'Asta V, Gazzola GC, Gatti R, Bussolati O. (2006) PKC-dependent stimulation of EAAT3 glutamate transporter does not require the integrity of actin cytoskeleton. Neurochem Int 48:341–349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

