Agonist-regulated cleavage of the extracellular domain of parathyroid hormone receptor type 1
- PMID: 20080964
- PMCID: PMC2838289
- DOI: 10.1074/jbc.M109.058685
Agonist-regulated cleavage of the extracellular domain of parathyroid hormone receptor type 1
Abstract
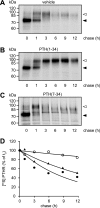
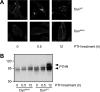
The receptor for parathyroid hormone (PTHR) is a main regulator of calcium homeostasis and bone maintenance. As a member of class B of G protein-coupled receptors, it harbors a large extracellular domain, which is required for ligand binding. Here, we demonstrate that the PTHR extracellular domain is cleaved by a protease belonging to the family of extracellular metalloproteinases. We show that the cleavage takes place in a region of the extracellular domain that belongs to an unstructured loop connecting the ligand-binding parts and that the N-terminal 10-kDa fragment is connected to the receptor core by a disulfide bond. Cleaved receptor revealed reduced protein stability compared with noncleaved receptor, suggesting degradation of the whole receptor. In the presence of the agonistic peptides PTH(1-34), PTH(1-14), or PTH(1-31), the processing of the PTHR extracellular domain was inhibited, and receptor protein levels were stabilized. A processed form of the PTHR was also detected in human kidney. These findings suggest a new model of PTHR processing and regulation of its stability.
Figures






Similar articles
-
Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation.Proc Natl Acad Sci U S A. 2008 Oct 28;105(43):16525-30. doi: 10.1073/pnas.0808750105. Epub 2008 Oct 22. Proc Natl Acad Sci U S A. 2008. PMID: 18946036 Free PMC article.
-
Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism.Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16084-9. doi: 10.1073/pnas.0503942102. Epub 2005 Oct 18. Proc Natl Acad Sci U S A. 2005. PMID: 16236727 Free PMC article.
-
Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling.J Biol Chem. 2016 May 20;291(21):10986-1002. doi: 10.1074/jbc.M115.697045. Epub 2016 Mar 23. J Biol Chem. 2016. PMID: 27008860 Free PMC article.
-
PTH/PTHrP Receptor Signaling, Allostery, and Structures.Trends Endocrinol Metab. 2019 Nov;30(11):860-874. doi: 10.1016/j.tem.2019.07.011. Trends Endocrinol Metab. 2019. PMID: 31699241 Free PMC article. Review.
-
Structural characterization of the parathyroid hormone receptor domains determinant for ligand binding.Biochem Soc Trans. 2007 Aug;35(Pt 4):721-3. doi: 10.1042/BST0350721. Biochem Soc Trans. 2007. PMID: 17635133 Review.
Cited by
-
High-resolution crystal structure of parathyroid hormone 1 receptor in complex with a peptide agonist.Nat Struct Mol Biol. 2018 Dec;25(12):1086-1092. doi: 10.1038/s41594-018-0151-4. Epub 2018 Nov 19. Nat Struct Mol Biol. 2018. PMID: 30455434
-
GPR37 is processed in the N-terminal ectodomain by ADAM10 and furin.FASEB J. 2021 Jun;35(6):e21654. doi: 10.1096/fj.202002385RR. FASEB J. 2021. PMID: 34042202 Free PMC article.
-
MMP14 cleaves PTH1R in the chondrocyte-derived osteoblast lineage, curbing signaling intensity for proper bone anabolism.Elife. 2023 Mar 9;12:e82142. doi: 10.7554/eLife.82142. Elife. 2023. PMID: 36892459 Free PMC article.
-
SheddomeDB: the ectodomain shedding database for membrane-bound shed markers.BMC Bioinformatics. 2017 Mar 14;18(Suppl 3):42. doi: 10.1186/s12859-017-1465-7. BMC Bioinformatics. 2017. PMID: 28361715 Free PMC article. Review.
-
ADAM19 cleaves the PTH receptor and associates with brachydactyly type E.Life Sci Alliance. 2024 Feb 8;7(4):e202302400. doi: 10.26508/lsa.202302400. Print 2024 Apr. Life Sci Alliance. 2024. PMID: 38331475 Free PMC article.
References
-
- Potts J. T. (2005) J. Endocrinol. 187, 311–325 - PubMed
-
- Neer R. M., Arnaud C. D., Zanchetta J. R., Prince R., Gaich G. A., Reginster J. Y., Hodsman A. B., Eriksen E. F., Ish-Shalom S., Genant H. K., Wang O., Mitlak B. H. (2001) N. Engl. J. Med. 344, 1434–1441 - PubMed
-
- Foord S. M., Bonner T. I., Neubig R. R., Rosser E. M., Pin J. P., Davenport A. P., Spedding M., Harmar A. J. (2005) Pharmacol. Rev. 57, 279–288 - PubMed
-
- Pines M., Fukayama S., Costas K., Meurer E., Goldsmith P. K., Xu X., Muallem S., Behar V., Chorev M., Rosenblatt M., Tashjian A. H., Jr., Suva L. J. (1996) Bone 18, 381–389 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

