Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin
- PMID: 20080971
- PMCID: PMC2838306
- DOI: 10.1074/jbc.M109.053934
Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin
Abstract
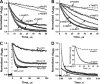
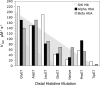
The role of the distal histidine in regulating ligand binding to adult human hemoglobin (HbA) was re-examined systematically by preparing His(E7) to Gly, Ala, Leu, Gln, Phe, and Trp mutants of both Hb subunits. Rate constants for O(2), CO, and NO binding were measured using rapid mixing and laser photolysis experiments designed to minimize autoxidation of the unstable apolar E7 mutants. Replacing His(E7) with Gly, Ala, Leu, or Phe causes 20-500-fold increases in the rates of O(2) dissociation from either Hb subunit, demonstrating unambiguously that the native His(E7) imidazole side chain forms a strong hydrogen bond with bound O(2) in both the alpha and beta chains (DeltaG(His(E7)H-bond) approximately -8 kJ/mol). As the size of the E7 amino acid is increased from Gly to Phe, decreases in k(O2)', k(NO)', and calculated bimolecular rates of CO entry (k(entry)') are observed. Replacing His(E7) with Trp causes further decreases in k(O2)', k(NO)', and k(entry)' to 1-2 microM(-1) s(-1) in beta subunits, whereas ligand rebinding to alphaTrp(E7) subunits after photolysis is markedly biphasic, with fast k(O2)', k(CO)', and k(NO)' values approximately 150 microM(-1) s(-1) and slow rate constants approximately 0.1 to 1 microM(-1) s(-1). Rapid bimolecular rebinding to an open alpha subunit conformation occurs immediately after photolysis of the alphaTrp(E7) mutant at high ligand concentrations. However, at equilibrium the closed alphaTrp(E7) side chain inhibits the rate of ligand binding >200-fold. These data suggest strongly that the E7 side chain functions as a gate for ligand entry in both HbA subunits.
Figures
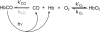
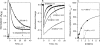
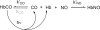
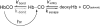
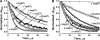
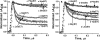
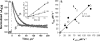
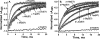


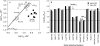
References
-
- Perutz M. F. (1970) Nature 228, 726–739 - PubMed
-
- Pauling L. (1964) Nature 203, 182–183
-
- Perutz M. F., Mathews F. S. (1966) J. Mol. Biol. 21, 199–202 - PubMed
-
- Jameson G. B., Molinaro F. S., Ibers J. A., Collman J. P., Brauman J. I., Rose E., Suslick K. S. (1980) J. Am. Chem. Soc. 102, 3224–3237
-
- Slebodnick C., Ibers J. A. (1997) J. Biol. Inorg. Chem. 2, 521–525
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

