Arginine methylation of vasa protein is conserved across phyla
- PMID: 20080973
- PMCID: PMC2832966
- DOI: 10.1074/jbc.M109.089821
Arginine methylation of vasa protein is conserved across phyla
Abstract
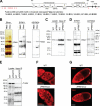
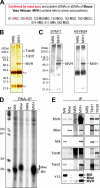
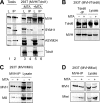
Recent studies have uncovered an unexpected relationship between factors that are essential for germline development in Drosophila melanogaster: the arginine protein methyltransferase 5 (dPRMT5/Csul/Dart5) and its cofactor Valois, methylate the Piwi family protein Aub, enabling it to bind Tudor. The RNA helicase Vasa is another essential protein in germline development. Here, we report that mouse (mouse Vasa homolog), Xenopus laevis, and D. melanogaster Vasa proteins contain both symmetrical and asymmetrical dimethylarginines. We find that dPRMT5 is required for the production of sDMAs of Vasa in vivo. Furthermore, we find that the mouse Vasa homolog associates with Tudor domain-containing proteins, Tdrd1 and Tdrd6, as well as the Piwi proteins, Mili and Miwi. Arginine methylation is thus emerging as a conserved and pivotal post-translational modification of proteins that is essential for germline development.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

