RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium
- PMID: 20081028
- PMCID: PMC2832520
- DOI: 10.1128/JB.01517-09
RamA, a member of the AraC/XylS family, influences both virulence and efflux in Salmonella enterica serovar Typhimurium
Abstract
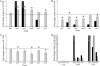
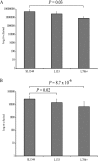
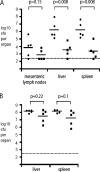
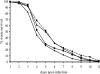
The transcriptomes of Salmonella enterica serovar Typhimurium SL1344 lacking a functional ramA or ramR or with plasmid-mediated high-level overexpression of ramA were compared to those of the wild-type parental strain. Inactivation of ramA led to increased expression of 14 SPI-1 genes and decreased expression of three SPI-2 genes, and it altered expression of ribosomal biosynthetic genes and several amino acid biosynthetic pathways. Furthermore, disruption of ramA led to decreased survival within RAW 264.7 mouse macrophages and attenuation within the BALB/c ByJ mouse model. Highly overexpressed ramA led to increased expression of genes encoding multidrug resistance (MDR) efflux pumps, including acrAB, acrEF, and tolC. Decreased expression of 34 Salmonella pathogenicity island (SPI) 1 and 2 genes, decreased SipC production, decreased adhesion to and survival within macrophages, and decreased colonization of Caenorhabditis elegans were also seen. Disruption of ramR led to the increased expression of ramA, acrAB, and tolC, but not to the same level as when ramA was overexpressed on a plasmid. Inactivation of ramR had a more limited effect on pathogenicity gene expression. In silico analysis of a suggested RamA-binding consensus sequence identified target genes, including ramR, acrA, tolC, sipABC, and ssrA. This study demonstrates that the regulation of a mechanism of MDR and expression of virulence genes show considerable overlap, and we postulate that such a mechanism is dependent on transcriptional activator concentration and promoter sensitivity. However, we have no evidence to support the hypothesis that increased MDR via RamA regulation of AcrAB-TolC gives rise to a hypervirulent strain.
Figures


References
-
- Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

