Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury
- PMID: 20081052
- PMCID: PMC2993087
- DOI: 10.1165/rcmb.2009-0345OC
Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury
Abstract
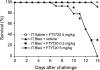
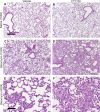
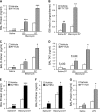
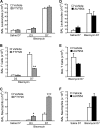
Sphingosine 1-phosphate (S1P) is a key endogenous regulator of the response to lung injury, maintaining endothelial barrier integrity through interaction with one of its receptors, S1P(1). The short-term administration of S1P or S1P(1) receptor agonists enhances endothelial monolayer barrier function in vitro, and attenuates injury-induced vascular leak in the lung and other organ systems in vivo. Although S1P(1) agonists bind to and activate S1P(1), several of these agents also induce receptor internalization and degradation, and may therefore act as functional antagonists of S1P(1) after extended exposure. Here we report on the effects of prolonged exposure to these agents in bleomycin-induced lung injury. We demonstrate that repeated administration of S1P(1) agonists dramatically worsened lung injury after bleomycin challenge, as manifested by increased vascular leak and mortality. Consistent with these results, prolonged exposure to S1P(1) agonists in vitro eliminated the ability of endothelial cell monolayers to respond appropriately to the barrier-protective effects of S1P, indicating a loss of normal S1P-S1P(1) signaling. As bleomycin-induced lung injury progressed, continued exposure to S1P(1) agonists also resulted in increased pulmonary fibrosis. These data indicate that S1P(1) agonists can act as functional antagonists of S1P(1) on endothelial cells in vivo, which should be considered in developing these agents as therapies for vascular leak syndromes. Our findings also support the hypothesis that vascular leak is an important component of the fibrogenic response to lung injury, and suggest that targeting the S1P-S1P(1) pathway may also be an effective therapeutic strategy for fibrotic lung diseases.
Figures
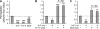
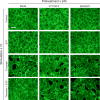
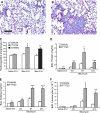
References
-
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1–phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998;279:1552–1555. - PubMed
-
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 2004;73:321–354. - PubMed
-
- Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 2008;160:25–46. - PubMed
-
- Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1–phosphates as bisacetylated derivatives by liquid chromatography–tandem mass spectrometry. Anal Biochem 2005;339:129–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

