L718P mutation in the membrane-proximal cytoplasmic tail of beta 3 promotes abnormal alpha IIb beta 3 clustering and lipid microdomain coalescence, and associates with a thrombasthenia-like phenotype
- PMID: 20081061
- PMCID: PMC2895041
- DOI: 10.3324/haematol.2009.018572
L718P mutation in the membrane-proximal cytoplasmic tail of beta 3 promotes abnormal alpha IIb beta 3 clustering and lipid microdomain coalescence, and associates with a thrombasthenia-like phenotype
Abstract
Background: Support for the role of transmembrane and membrane-proximal domains of alpha IIb beta 3 integrin in the maintenance of receptor low affinity comes from mutational studies showing that activating mutations can induce constitutive bi-directional transmembrane signaling.
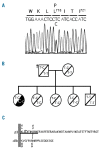
Design and methods: We report the functional characterization of a mutant alpha IIb beta 3 integrin carrying the Leu718Pro mutation in the membrane-proximal region of the beta 3 cytoplasmic domain, identified in heterozygosis in a patient with a severe bleeding phenotype and defective platelet aggregation and adhesion.
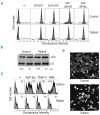
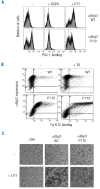
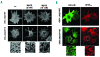
Results: Transiently transfected cells expressed similar levels of normal and mutant alpha IIb beta 3, but surface expression of mutant alpha v beta 3 was reduced due to its retention in intracellular compartments. Cells stably expressing mutant alpha IIb beta 3 showed constitutive binding to soluble multivalent ligands as well as spontaneous fibrinogen-dependent aggregation, but their response to DTT was markedly reduced. Fibrinogen-adherent cells exhibited a peculiar spreading phenotype with long protrusions. Immunofluorescence analysis revealed the formation of alpha IIb beta 3 clusters underneath the entire cell body and the presence of atypical high-density patches of clustered alpha IIb beta 3 containing encircled areas devoid of integrin that showed decreased affinity for the fluorescent lipid analog DiIC(16) and were disrupted in cholesterol-depleted cells.
Conclusions: These findings are consistent with an important role of the membrane-proximal region of beta 3 in modulating alpha IIb beta 3 clustering and lateral redistribution of membrane lipids. Since the beta 3 mutant was associated with a thrombasthenic phenotype in a patient carrying one normal beta 3 allele, these results support a dominant role of clustering in regulating integrin alpha IIb beta 3 functions in vivo.
Figures


Comment in
-
Regulation of platelet beta 3 integrins.Haematologica. 2010 Jul;95(7):1049-51. doi: 10.3324/haematol.2010.024893. Haematologica. 2010. PMID: 20595101 Free PMC article. Review. No abstract available.
Similar articles
-
A mutation in the β3 cytoplasmic tail causes variant Glanzmann thrombasthenia by abrogating transition of αIIb β3 to an active state.J Thromb Haemost. 2012 Feb;10(2):289-97. doi: 10.1111/j.1538-7836.2011.04577.x. J Thromb Haemost. 2012. PMID: 22136613
-
Ser-752-->Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia.Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10169-73. doi: 10.1073/pnas.89.21.10169. Proc Natl Acad Sci U S A. 1992. PMID: 1438206 Free PMC article.
-
Abnormal cytoplasmic extensions associated with active αIIbβ3 are probably the cause for macrothrombocytopenia in Glanzmann thrombasthenia-like syndrome.Blood Coagul Fibrinolysis. 2015 Apr;26(3):302-8. doi: 10.1097/MBC.0000000000000241. Blood Coagul Fibrinolysis. 2015. PMID: 25806962
-
Glanzmann thrombasthenia: integrin alpha IIb beta 3 deficiency.Int J Hematol. 2000 Dec;72(4):448-54. Int J Hematol. 2000. PMID: 11197210 Review.
-
Glanzmann thrombasthenia: genetic basis and clinical correlates.Haematologica. 2020 Apr;105(4):888-894. doi: 10.3324/haematol.2018.214239. Epub 2020 Mar 5. Haematologica. 2020. PMID: 32139434 Free PMC article. Review.
Cited by
-
Structural and thermodynamic basis of proline-induced transmembrane complex stabilization.Sci Rep. 2016 Jul 20;6:29809. doi: 10.1038/srep29809. Sci Rep. 2016. PMID: 27436065 Free PMC article.
-
A novel variant Glanzmann thrombasthenia due to co-inheritance of a loss- and a gain-of-function mutation of ITGB3: evidence of a dominant effect of gain-of-function mutations.Haematologica. 2018 Jun;103(6):e259-e263. doi: 10.3324/haematol.2017.180927. Epub 2018 Feb 8. Haematologica. 2018. PMID: 29439184 Free PMC article. No abstract available.
-
αIIbβ3 variants defined by next-generation sequencing: predicting variants likely to cause Glanzmann thrombasthenia.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1898-907. doi: 10.1073/pnas.1422238112. Epub 2015 Mar 31. Proc Natl Acad Sci U S A. 2015. PMID: 25827233 Free PMC article.
-
Profiling the Genetic and Molecular Characteristics of Glanzmann Thrombasthenia: Can It Guide Current and Future Therapies?J Blood Med. 2021 Jul 8;12:581-599. doi: 10.2147/JBM.S273053. eCollection 2021. J Blood Med. 2021. PMID: 34267570 Free PMC article. Review.
-
Demonstration of novel gain-of-function mutations of αIIbβ3: association with macrothrombocytopenia and glanzmann thrombasthenia-like phenotype.Mol Genet Genomic Med. 2013 Jul;1(2):77-86. doi: 10.1002/mgg3.9. Epub 2013 Apr 22. Mol Genet Genomic Med. 2013. PMID: 24498605 Free PMC article.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. - PubMed
-
- Partridge AW, Liu S, Kim S, Bowie JU, Ginsberg MH. Transmembrane domain helix packing stabilizes integrin αIIbβ3 in the low affinity state. J Biol Chem. 2005;280(8):7294–300. - PubMed
-
- Hughes P, O’Toole TE, Ylänne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J Biol Chem. 1995;270(21):12411–7. - PubMed
-
- Ma YQ, Yang J, Pesho MM, Vinogradova O, Qin J, Plow EF. Regulation of integrin alphaIIbbeta3 activation by distinct regions of its cytoplasmic tails. Biochemistry. 2006;45(21):6656–62. - PubMed

