Insights into the organization of dorsal spinal cord pathways from an evolutionarily conserved raldh2 intronic enhancer
- PMID: 20081195
- PMCID: PMC4074295
- DOI: 10.1242/dev.043257
Insights into the organization of dorsal spinal cord pathways from an evolutionarily conserved raldh2 intronic enhancer
Abstract
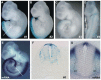
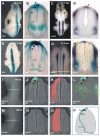
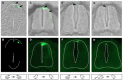
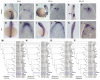
Comparative studies of the tetrapod raldh2 (aldh1a2) gene, which encodes a retinoic acid (RA) synthesis enzyme, have led to the identification of a dorsal spinal cord enhancer. Enhancer activity is directed dorsally to the roof plate and dorsal-most (dI1) interneurons through predicted Tcf- and Cdx-homeodomain binding sites and is repressed ventrally via predicted Tgif homeobox and ventral Lim-homeodomain binding sites. Raldh2 and Math1/Cath1 expression in mouse and chicken highlights a novel, transient, endogenous Raldh2 expression domain in dI1 interneurons, which give rise to ascending circuits and intraspinal commissural interneurons, suggesting roles for RA in the ontogeny of spinocerebellar and intraspinal proprioceptive circuits. Consistent with expression of raldh2 in the dorsal interneurons of tetrapods, we also found that raldh2 is expressed in dorsal interneurons throughout the agnathan spinal cord, suggesting ancestral roles for RA signaling in the ontogenesis of intraspinal proprioception.
Figures




Similar articles
-
Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development.Dev Dyn. 2005 Apr;232(4):950-7. doi: 10.1002/dvdy.20256. Dev Dyn. 2005. PMID: 15739227
-
Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice.Dev Biol. 2005 Aug 15;284(2):399-411. doi: 10.1016/j.ydbio.2005.05.035. Dev Biol. 2005. PMID: 16026781
-
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice.Development. 2002 May;129(9):2271-82. doi: 10.1242/dev.129.9.2271. Development. 2002. PMID: 11959834 Free PMC article.
-
Getting in touch with your senses: Mechanisms specifying sensory interneurons in the dorsal spinal cord.WIREs Mech Dis. 2021 Sep;13(5):e1520. doi: 10.1002/wsbm.1520. Epub 2021 Feb 25. WIREs Mech Dis. 2021. PMID: 34730293 Free PMC article. Review.
-
The spinal cord shows the way - How axons navigate intermediate targets.Dev Biol. 2017 Dec 1;432(1):43-52. doi: 10.1016/j.ydbio.2016.12.002. Epub 2016 Dec 10. Dev Biol. 2017. PMID: 27965053 Review.
Cited by
-
Completion of neural crest cell production and emigration is regulated by retinoic-acid-dependent inhibition of BMP signaling.Elife. 2022 Apr 8;11:e72723. doi: 10.7554/eLife.72723. Elife. 2022. PMID: 35394423 Free PMC article.
-
Link between the causative genes of holoprosencephaly: Zic2 directly regulates Tgif1 expression.Sci Rep. 2018 Feb 1;8(1):2140. doi: 10.1038/s41598-018-20242-2. Sci Rep. 2018. PMID: 29391420 Free PMC article.
-
Signaling through retinoic acid receptors in cardiac development: Doing the right things at the right times.Biochim Biophys Acta. 2015 Feb;1849(2):94-111. doi: 10.1016/j.bbagrm.2014.08.003. Epub 2014 Aug 15. Biochim Biophys Acta. 2015. PMID: 25134739 Free PMC article. Review.
-
Inhibition of GSK3 Represses the Expression of Retinoic Acid Synthetic Enzyme ALDH1A2 via Wnt/β-Catenin Signaling in WiT49 Cells.Front Cell Dev Biol. 2020 Mar 17;8:94. doi: 10.3389/fcell.2020.00094. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32258025 Free PMC article.
-
Sea lamprey enlightens the origin of the coupling of retinoic acid signaling to vertebrate hindbrain segmentation.Nat Commun. 2024 Feb 20;15(1):1538. doi: 10.1038/s41467-024-45911-x. Nat Commun. 2024. PMID: 38378737 Free PMC article.
References
-
- Abascal F., Zardoya R., Posada D. (2005). ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 21-2104 - PubMed
-
- Altman J., Bayer S. A. (1997). Development of the Cerebellar System In Relation to Its Evolution, Structure, and Functions. New York: CRC Press;
-
- Berggren K., McCaffery P., Drager U., Forehand C. J. (1999). Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev. Biol. 210, 210-288 - PubMed
-
- Bermingham N. A., Hassan B. A., Wang V. Y., Fernandez M., Banfi S., Bellen H. J., Fritzsch B., Zoghbi H. Y. (2001). Proprioceptor pathway development is dependent on Math1. Neuron 30, 30-411 - PubMed
-
- Blentic A., Gale E., Maden M. (2003). Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev. Dyn. 227, 227-114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

