The Engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum
- PMID: 20081196
- PMCID: PMC2858911
- DOI: 10.1242/dev.027045
The Engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum
Abstract
Little is known about the genetic pathways and cellular processes responsible for regional differences in cerebellum foliation, which interestingly are accompanied by regionally distinct afferent circuitry. We have identified the Engrailed (En) homeobox genes as being crucial to producing the distinct medial vermis and lateral hemisphere foliation patterns in mammalian cerebella. By producing a series of temporal conditional mutants in En1 and/or En2, we demonstrate that both En genes are required to ensure that folia exclusive to the vermis or hemispheres form in the appropriate mediolateral position. Furthermore, En1/En2 continue to regulate foliation after embryonic day 14, at which time Fgf8 isthmic organizer activity is complete and the major output cells of the cerebellar cortex have been specified. Changes in spatially restricted gene expression occur prior to foliation in mutants, and foliation is altered from the onset and is accompanied by changes in the thickness of the layer of proliferating granule cell precursors. In addition, the positioning and timing of fissure formation are altered. Thus, the En genes represent a new class of genes that are fundamental to patterning cerebellum foliation throughout the mediolateral axis and that act late in development.
Figures


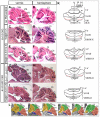




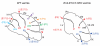
Similar articles
-
Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum.J Neurosci. 2008 Nov 19;28(47):12150-62. doi: 10.1523/JNEUROSCI.2059-08.2008. J Neurosci. 2008. PMID: 19020009 Free PMC article.
-
Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern.Neural Dev. 2016 Sep 8;11(1):17. doi: 10.1186/s13064-016-0072-z. Neural Dev. 2016. PMID: 27609139 Free PMC article.
-
Genetic subdivision of the tectum and cerebellum into functionally related regions based on differential sensitivity to engrailed proteins.Development. 2007 Jun;134(12):2325-35. doi: 10.1242/dev.000620. Development. 2007. PMID: 17537797 Free PMC article.
-
Neurogenetics of the cerebellar system.J Child Neurol. 1999 Sep;14(9):574-81; discussion 581-2. doi: 10.1177/088307389901400905. J Child Neurol. 1999. PMID: 10488902 Review.
-
Abnormalities of cerebellar foliation and fissuration: classification, neurogenetics and clinicoradiological correlations.Neuroradiology. 2002 Aug;44(8):639-46. doi: 10.1007/s00234-002-0783-1. Epub 2002 Jun 26. Neuroradiology. 2002. PMID: 12185541 Review.
Cited by
-
Principal component and cluster analysis of morphological variables reveals multiple discrete sub-phenotypes in weaver mouse mutants.Cerebellum. 2013 Jun;12(3):406-17. doi: 10.1007/s12311-012-0429-8. Cerebellum. 2013. PMID: 23179325
-
Clinical Phenotypes Associated to Engrailed 2 Gene Alterations in a Series of Neuropediatric Patients.Front Neuroanat. 2018 Aug 10;12:61. doi: 10.3389/fnana.2018.00061. eCollection 2018. Front Neuroanat. 2018. PMID: 30147646 Free PMC article.
-
Conditional loss of Engrailed1/2 in Atoh1-derived excitatory cerebellar nuclear neurons impairs eupneic respiration in mice.Genes Brain Behav. 2022 Feb;21(2):e12788. doi: 10.1111/gbb.12788. Epub 2022 Jan 19. Genes Brain Behav. 2022. PMID: 35044072 Free PMC article.
-
Dual analysis of the murine cytomegalovirus and host cell transcriptomes reveal new aspects of the virus-host cell interface.PLoS Pathog. 2013;9(9):e1003611. doi: 10.1371/journal.ppat.1003611. Epub 2013 Sep 26. PLoS Pathog. 2013. PMID: 24086132 Free PMC article.
-
Elevated 5-hydroxymethylcytosine in the Engrailed-2 (EN-2) promoter is associated with increased gene expression and decreased MeCP2 binding in autism cerebellum.Transl Psychiatry. 2014 Oct 7;4(10):e460. doi: 10.1038/tp.2014.87. Transl Psychiatry. 2014. PMID: 25290267 Free PMC article.
References
-
- Altman J. and Bayer, S. A. (1997). Development of the Cerebellar System in Relation to its Evolution, Structure, and Functions. New York: CRC Press.
-
- Bai C. B., Auerbach, W., Lee, J. S., Stephen, D. and Joyner, A. L. (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 129-4753. - PubMed
-
- Broccoli V, Boncinelli, E. and Wurst, W. (1999). The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401, 401-164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

