Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation
- PMID: 20081197
- PMCID: PMC2838346
- DOI: 10.1074/jbc.M109.054213
Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation
Abstract
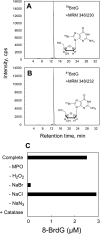
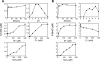
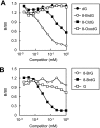
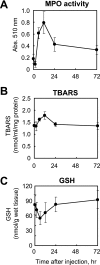
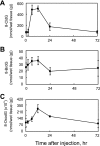
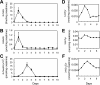
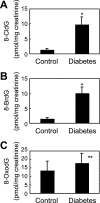
Myeloperoxidase (MPO) generates reactive halogenating species that can modify DNA. The aim of this study was to investigate the formation of 8-halogenated 2'-deoxyguanosines (8- halo-dGs) during inflammatory events. 8-Bromo-2'-dG (8-BrdG) and 8-chloro-2'-dG (8-CldG) were generated by treatment of MPO with hydrogen peroxide at physiological concentrations of Cl(-) and Br(-). The formation of 8-halo-dGs with other oxidative stress biomarkers in lipopolysaccharide-treated rats was assessed by liquid chromatography tandem mass spectrometry and immunohistochemistry using a novel monoclonal antibody (mAb8B3) to 8-BrdG-conjugated keyhole limpet hemocyanin. The antibody recognized both 8-BrdG and 8-CldG. In the liver of lipopolysaccharide-treated rats, immunostaining for 8-halo-dGs, halogenated tyrosines, and MPO were increased at 8 h, whereas those of 8-oxo-2'-dG (8-OxodG) and 3-nitrotyrosine were increased at 24 h. Urinary excretion of both 8-CldG and 8-BrdG was also observed earlier than those of 8-OxodG and modified tyrosines (3-nitrotyrosine, 3-chlorotyrosine, and 3- bromotyrosine). Moreover, the levels of the 8-halo-dGs in urine from human diabetic patients were 8-fold higher than in healthy subjects (n = 10, healthy and diabetic, p < 0.0001), whereas there was a moderate difference in 8-OxodG between the two groups (p < 0.001). Interestingly, positive mAb8B3 antibody staining was observed in liver tissue from hepatocellular carcinoma patients but not in liver tissue from human cirrhosis patients. These data suggest that 8-halo-dGs may be potential biomarkers of early inflammation.
Figures



References
-
- Ohshima H., Bartsch H. (1994) Mutat. Res. 305, 253–264 - PubMed
-
- Takeshita J., Byun J., Nhan T. Q., Pritchard D. K., Pennathur S., Schwartz S. M., Chait A., Heinecke J. W. (2006) J. Biol. Chem. 281, 3096–3104 - PubMed
-
- Weitzman S. A., Gordon L. I. (1990) Blood 76, 655–663 - PubMed
-
- Henderson J. P., Byun J., Heinecke J. W. (1999) J. Biol. Chem. 274, 33440–33448 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

