Regulation of Akt signaling activation by ubiquitination
- PMID: 20081374
- PMCID: PMC3077544
- DOI: 10.4161/cc.9.3.10508
Regulation of Akt signaling activation by ubiquitination
Abstract
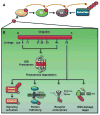
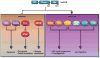
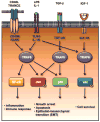
Akt (also known as PKB) signaling orchestrates many aspects of biological functions and, importantly, its deregulation is linked to cancer development. Akt activity is well-known regulated through its phosphorylation at T308 and S473 by PDK1 and mTOrC2, respectively. Although in the last decade the research has been primarily focused on Akt phosphorylation and its role in Akt activation and functions, other posttranslational modifications on Akt have never been reported. Until very recently, a novel posttranslational modification on Akt termed ubiquitination was identified and shown to play an important role in Akt activation. The cancer-associated Akt mutant recently identified in a subset of human cancers displays enhanced Akt ubiquitination, in turn contributing to Akt hyperactivation, suggesting a potential role of Akt ubiquitination in cancers. Thus, this novel posttranslational modification on Akt reveals an exciting avenue that has advanced our current understandings of how Akt signaling activation is regulated.
Figures


References
-
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. - PubMed
-
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–81. - PubMed
-
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
