Chemoselective small molecules that covalently modify one lysine in a non-enzyme protein in plasma
- PMID: 20081815
- PMCID: PMC3107129
- DOI: 10.1038/nchembio.281
Chemoselective small molecules that covalently modify one lysine in a non-enzyme protein in plasma
Abstract
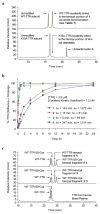
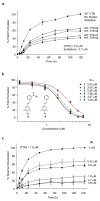
A small molecule that could bind selectively to and then react chemoselectively with a non-enzyme protein in a complex biological fluid, such as blood, could have numerous practical applications. Herein, we report a family of designed stilbenes that selectively and covalently modify the prominent plasma protein transthyretin in preference to more than 4,000 other human plasma proteins. They react chemoselectively with only one of eight lysine e-amino groups within transthyretin. The crystal structure confirms the expected binding orientation of the stilbene substructure and the anticipated conjugating amide bond. These covalent transthyretin kinetic stabilizers exhibit superior amyloid inhibition potency compared to their noncovalent counterparts, and they prevent cytotoxicity associated with amyloidogenesis. Though there are a few prodrugs that, upon metabolic activation, react with a cysteine residue inactivating a specific non-enzyme, we are unaware of designed small molecules that react with one lysine e-amine within a specific non-enzyme protein in a complex biological fluid.
Conflict of interest statement
JWK is a founder, shareholder and paid consultant for Foldrx Pharmaceuticals, Inc., a biotechnology company that specializes in the discovery and development of drug therapies for transthyretin amyloidoses.
Figures

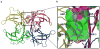
Similar articles
-
Stilbene vinyl sulfonamides as fluorogenic sensors of and traceless covalent kinetic stabilizers of transthyretin that prevent amyloidogenesis.J Am Chem Soc. 2013 Nov 27;135(47):17869-80. doi: 10.1021/ja408230k. Epub 2013 Nov 18. J Am Chem Soc. 2013. PMID: 24180271 Free PMC article.
-
A substructure combination strategy to create potent and selective transthyretin kinetic stabilizers that prevent amyloidogenesis and cytotoxicity.J Am Chem Soc. 2010 Feb 3;132(4):1359-70. doi: 10.1021/ja908562q. J Am Chem Soc. 2010. PMID: 20043671 Free PMC article.
-
A competition assay to identify amyloidogenesis inhibitors by monitoring the fluorescence emitted by the covalent attachment of a stilbene derivative to transthyretin.Bioorg Med Chem. 2011 Feb 15;19(4):1505-14. doi: 10.1016/j.bmc.2010.12.050. Epub 2010 Dec 30. Bioorg Med Chem. 2011. PMID: 21273081 Free PMC article.
-
Structure-based design of kinetic stabilizers that ameliorate the transthyretin amyloidoses.Curr Opin Struct Biol. 2010 Feb;20(1):54-62. doi: 10.1016/j.sbi.2009.12.009. Epub 2010 Feb 3. Curr Opin Struct Biol. 2010. PMID: 20133122 Free PMC article. Review.
-
Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses.Acc Chem Res. 2005 Dec;38(12):911-21. doi: 10.1021/ar020073i. Acc Chem Res. 2005. PMID: 16359163 Review.
Cited by
-
β-Glucocerebrosidase Modulators Promote Dimerization of β-Glucocerebrosidase and Reveal an Allosteric Binding Site.J Am Chem Soc. 2018 May 9;140(18):5914-5924. doi: 10.1021/jacs.7b13003. Epub 2018 Apr 30. J Am Chem Soc. 2018. PMID: 29676907 Free PMC article.
-
Enhancing the Pharmacokinetic Profile of Interleukin 2 through Site-Specific Conjugation to a Selective Small-Molecule Transthyretin Ligand.J Med Chem. 2021 Oct 14;64(19):14876-14886. doi: 10.1021/acs.jmedchem.1c01426. Epub 2021 Sep 20. J Med Chem. 2021. PMID: 34542267 Free PMC article.
-
Peptide-Based Inhibitors of Fimbrial Biogenesis in Porphyromonas gingivalis.Infect Immun. 2019 Feb 21;87(3):e00750-18. doi: 10.1128/IAI.00750-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30642895 Free PMC article.
-
Stilbene vinyl sulfonamides as fluorogenic sensors of and traceless covalent kinetic stabilizers of transthyretin that prevent amyloidogenesis.J Am Chem Soc. 2013 Nov 27;135(47):17869-80. doi: 10.1021/ja408230k. Epub 2013 Nov 18. J Am Chem Soc. 2013. PMID: 24180271 Free PMC article.
-
Cellular clearance of circulating transthyretin decreases cell-nonautonomous proteotoxicity in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7710-E7719. doi: 10.1073/pnas.1801117115. Epub 2018 Jul 30. Proc Natl Acad Sci U S A. 2018. PMID: 30061394 Free PMC article.
References
-
- Savi P, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. - PubMed
-
- Estebanez-Perpina E, et al. Structural insight into the mode of action of a direct inhibitor of coregulator binding to the thyroid hormone receptor. Mol Endocrinol. 2007;21:2919–2928. - PubMed
-
- Cohen E, et al. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

