An in vitro translation, selection and amplification system for peptide nucleic acids
- PMID: 20081830
- PMCID: PMC2808706
- DOI: 10.1038/nchembio.280
An in vitro translation, selection and amplification system for peptide nucleic acids
Abstract
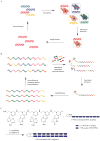
Methods to evolve synthetic, rather than biological, polymers could significantly expand the functional potential of polymers that emerge from in vitro evolution. Requirements for synthetic polymer evolution include (i) sequence-specific polymerization of synthetic building blocks on an amplifiable template, (ii) display of the newly translated polymer strand in a manner that allows it to adopt folded structures, (iii) selection of synthetic polymer libraries for desired binding or catalytic properties and (iv) amplification of template sequences that survive selection in a manner that allows subsequent translation. Here we report the development of such a system for peptide nucleic acids (PNAs) using a set of 12 PNA pentamer building blocks. We validated the system by performing six iterated cycles of translation, selection and amplification on a library of 4.3 x 10(8) PNA-encoding DNA templates and observed >1,000,000-fold overall enrichment of a template encoding a biotinylated (streptavidin-binding) PNA. These results collectively provide an experimental foundation for PNA evolution in the laboratory.
Conflict of interest statement
Y.B. and D.R.L. are co-inventors on a Harvard University patent describing DNA-templated polymerization.
Figures






Comment in
-
Directed evolution: overcoming biology's limitations.Nat Chem Biol. 2010 Feb;6(2):87-8. doi: 10.1038/nchembio.300. Nat Chem Biol. 2010. PMID: 20081821 Free PMC article.
Similar articles
-
Pretargeting with amplification using polymeric peptide nucleic acid.Bioconjug Chem. 2001 Sep-Oct;12(5):807-16. doi: 10.1021/bc0100307. Bioconjug Chem. 2001. PMID: 11562199
-
DNA-templated polymerization of side-chain-functionalized peptide nucleic acid aldehydes.J Am Chem Soc. 2008 Apr 9;130(14):4646-59. doi: 10.1021/ja0753997. Epub 2008 Mar 15. J Am Chem Soc. 2008. PMID: 18341334 Free PMC article.
-
Enzyme-free translation of DNA into sequence-defined synthetic polymers structurally unrelated to nucleic acids.Nat Chem. 2013 Apr;5(4):282-92. doi: 10.1038/nchem.1577. Epub 2013 Mar 3. Nat Chem. 2013. PMID: 23511416 Free PMC article.
-
Peptide nucleic acid (PNA) binding-mediated gene regulation.Cell Res. 2004 Apr;14(2):111-6. doi: 10.1038/sj.cr.7290209. Cell Res. 2004. PMID: 15115611 Review.
-
Molecular self-assembly using peptide nucleic acids.Biopolymers. 2017 Jan;108(1):10.1002/bip.22930. doi: 10.1002/bip.22930. Biopolymers. 2017. PMID: 27486924 Free PMC article. Review.
Cited by
-
Rapid Chemical Ligation of DNA and Acyclic Threoninol Nucleic Acid (aTNA) for Effective Nonenzymatic Primer Extension.J Am Chem Soc. 2023 Aug 16;145(32):17872-17880. doi: 10.1021/jacs.3c04979. Epub 2023 Jul 19. J Am Chem Soc. 2023. PMID: 37466125 Free PMC article.
-
Aptamers for DNA Damage and Repair.Int J Mol Sci. 2017 Oct 22;18(10):2212. doi: 10.3390/ijms18102212. Int J Mol Sci. 2017. PMID: 29065503 Free PMC article. Review.
-
Strategies for developing DNA-encoded libraries beyond binding assays.Nat Chem. 2022 Feb;14(2):129-140. doi: 10.1038/s41557-021-00877-x. Epub 2022 Feb 4. Nat Chem. 2022. PMID: 35121833 Review.
-
Nonenzymatic polymerase-like template-directed synthesis of acyclic L-threoninol nucleic acid.Nat Commun. 2021 Feb 5;12(1):804. doi: 10.1038/s41467-021-21128-0. Nat Commun. 2021. PMID: 33547322 Free PMC article.
-
Multiplexed microcolumn-based process for efficient selection of RNA aptamers.Anal Chem. 2013 Mar 19;85(6):3417-24. doi: 10.1021/ac400105e. Epub 2013 Feb 27. Anal Chem. 2013. PMID: 23398198 Free PMC article.
References
-
- Orgel LE. Unnatural selection in chemical systems. Acc Chem Res. 1995;28:109–118. - PubMed
-
- Leitzel JC, Lynn DG. Template-directed ligation: from DNA towards different versatile templates. Chem Rec. 2001;1:53–62. - PubMed
-
- Keefe AD, Cload ST. SELEX with modified nucleotides. Curr Opin Chem Biol. 2008;12:448–456. - PubMed
-
- Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct. 2006;35:225–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

