Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis
- PMID: 20081841
- PMCID: PMC4580288
- DOI: 10.1038/ncb2015
Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis
Abstract
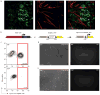
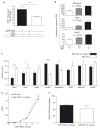
Efficient tissue regeneration is dependent on the coordinated responses of multiple cell types. Here, we describe a new subpopulation of fibro/adipogenic progenitors (FAPs) resident in muscle tissue but arising from a distinct developmental lineage. Transplantation of purified FAPs results in the generation of ectopic white fat when delivered subcutaneously or intramuscularly in a model of fatty infiltration, but not in healthy muscle, suggesting that the environment controls their engraftment. These cells are quiescent in intact muscle but proliferate efficiently in response to damage. FAPs do not generate myofibres, but enhance the rate of differentiation of primary myogenic progenitors in co-cultivation experiments. In summary, FAPs expand upon damage to provide a transient source of pro-differentiation signals for proliferating myogenic progenitors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Tipping the scale: muscle versus fat.Nat Cell Biol. 2010 Feb;12(2):102-4. doi: 10.1038/ncb0210-102. Epub 2010 Jan 17. Nat Cell Biol. 2010. PMID: 20081844
References
-
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. - PubMed
-
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. - PubMed
-
- Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

