Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin
- PMID: 20081860
- PMCID: PMC2850970
- DOI: 10.1038/ng.518
Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin
Abstract
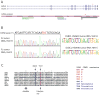
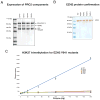
Follicular lymphoma (FL) and the GCB subtype of diffuse large B-cell lymphoma (DLBCL) derive from germinal center B cells. Targeted resequencing studies have revealed mutations in various genes encoding proteins in the NF-kappaB pathway that contribute to the activated B-cell (ABC) DLBCL subtype, but thus far few GCB-specific mutations have been identified. Here we report recurrent somatic mutations affecting the polycomb-group oncogene EZH2, which encodes a histone methyltransferase responsible for trimethylating Lys27 of histone H3 (H3K27). After the recent discovery of mutations in KDM6A (UTX), which encodes the histone H3K27me3 demethylase UTX, in several cancer types, EZH2 is the second histone methyltransferase gene found to be mutated in cancer. These mutations, which result in the replacement of a single tyrosine in the SET domain of the EZH2 protein (Tyr641), occur in 21.7% of GCB DLBCLs and 7.2% of FLs and are absent from ABC DLBCLs. Our data are consistent with the notion that EZH2 proteins with mutant Tyr641 have reduced enzymatic activity in vitro.
Figures
Comment in
-
Deregulation of H3K27 methylation in cancer.Nat Genet. 2010 Feb;42(2):100-1. doi: 10.1038/ng0210-100. Nat Genet. 2010. PMID: 20104248
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

