Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution
- PMID: 20081862
- PMCID: PMC2819359
- DOI: 10.1038/nm.2080
Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution
Abstract
Delayed myeloid engraftment after cord blood transplantation (CBT) is thought to result from inadequate numbers of progenitor cells in the graft and is associated with increased early transplant-related morbidity and mortality. New culture strategies that increase the number of cord blood progenitors capable of rapid myeloid engraftment after CBT would allow more widespread use of this stem cell source for transplantation. Here we report the development of a clinically relevant Notch-mediated ex vivo expansion system for human CD34(+) cord blood progenitors that results in a marked increase in the absolute number of stem/progenitor cells, including those capable of enhanced repopulation in the marrow of immunodeficient nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice. Furthermore, when cord blood progenitors expanded ex vivo in the presence of Notch ligand were infused in a clinical setting after a myeloablative preparative regimen for stem cell transplantation, the time to neutrophil recovery was substantially shortened. To our knowledge, this is the first instance of rapid engraftment derived from ex vivo expanded stem/progenitor cells in humans.
Conflict of interest statement
Figures
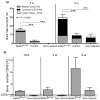
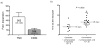
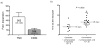
Comment in
-
Notch signaling: from stem cell expansion to improving cord blood transplantation.Expert Rev Hematol. 2010 Aug;3(4):401-4. doi: 10.1586/ehm.10.37. Expert Rev Hematol. 2010. PMID: 21083031
References
-
- Shpall E, et al. Transplantation of Ex Vivo Expanded Cord Blood. Biology of Blood and Marrow Transplantation. 2002;8:368–376. - PubMed
-
- Jaroscak J, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–7. - PubMed
-
- Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83:2057–62. - PubMed
-
- Varnum-Finney B, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

