Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials
- PMID: 20082144
- PMCID: PMC2935319
- DOI: 10.1245/s10434-009-0881-y
Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials
Abstract
Background: The objective of this study is to conduct a pooled analysis of National Surgical Adjuvant Breast and Bowel Project (NSABP) colon trials involving surgery and surgery plus 5-fluorouracil and leucovorin (5-FU/LV) to compare survival and establish a baseline from which to evaluate future studies.
Methods: All patients enrolled in NSABP adjuvant trials C-01 through C-05 with stage II and III disease who were treated with surgery or with surgery plus 5-FU/LV were examined for overall survival (OS), disease-free survival (DFS), and recurrence-free interval (RFI). Time-to-event by treatment group was examined using adjusted Kaplan-Meier estimates and multivariable Cox regression analysis.
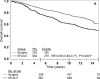
Results: There were 2,966 eligible patients: 693 (23%) surgery and 2,273 (77%) surgery plus 5-FU/LV; 1,255 (42%) stage II and 1,711 (58%) stage III. Age > or =60 years [hazard ratio (HR) = 1.36, P < 0.0001], male gender (HR = 1.20, P = 0.0012), and more nodes positive or fewer nodes examined (P < 0.0001) were associated with worse survival. At 5 years, the adjusted OS was 0.62 [confidence interval (CI) = 0.60-0.63] in the surgery group and 0.76 (CI = 0.74-0.78) in the surgery plus 5-FU/LV group. Treatment with 5-FU/LV was associated with improved outcome compared with surgery: OS (HR = 0.62, P < 0.0001), DFS (HR = 0.66, P < 0.0001) and RFI (HR = 0.64, P < 0.0001). Improved OS with adjuvant treatment was seen in both stage II (HR = 0.58, 95% CI = 0.48-0.71) and stage III disease (HR = 0.65, 95% CI = 0.55-0.75).
Conclusions: This analysis demonstrates that treatment of colon cancer patients with 5-FU/LV following surgery provides benefit over surgery alone and can provide anticipated survival outcomes with which to compare modern adjuvant trials.
Figures








Comment in
-
Adjuvant therapy for colon cancer: learning from the past to inform the future.Ann Surg Oncol. 2010 Apr;17(4):947-9. doi: 10.1245/s10434-009-0880-z. Ann Surg Oncol. 2010. PMID: 20094919 Free PMC article. No abstract available.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. Epub 2008 Feb 20. - PubMed
-
- Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–6. - PubMed
-
- Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–74. - PubMed
-
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–806. Epub 2004 Apr 5. - PubMed
-
- Heidelberger C, Chaudhuri NK, Danneberg P, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;30:663–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

