Opposing actions of insulin and arsenite converge on PKCdelta to alter keratinocyte proliferative potential and differentiation
- PMID: 20082316
- PMCID: PMC3152260
- DOI: 10.1002/mc.20612
Opposing actions of insulin and arsenite converge on PKCdelta to alter keratinocyte proliferative potential and differentiation
Abstract
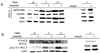
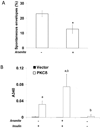
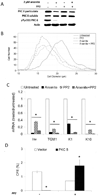
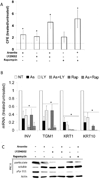
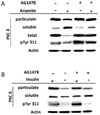
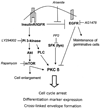
When cultured human keratinocytes reach confluence, they undergo a program of changes replicating features of differentiation in vivo, including exit from the proliferative pool, increased cell size, and expression of specialized differentiation marker proteins. Previously, we showed that insulin is required for some of these steps and that arsenite, a human carcinogen in skin and other epithelia, opposes the differentiation process. In present work, we show that insulin signaling, probably through the IGF-I receptor, is required for the increase in cell size accompanying differentiation and that this is opposed by arsenite. We further examine the impact of insulin and arsenite on PKCdelta, a known key regulator of keratinocyte differentiation, and show that insulin increases the amount, tyrosine phosphorylation, and membrane localization of PKCdelta. All these effects are prevented by exposure of cells to arsenite or to inhibitors of downstream effectors of insulin (phosphotidylinositol 3-kinase and mammalian target of rapamycin). Retrovirally mediated expression of activated PKCdelta resulted in increased loss of proliferative potential after confluence and greatly increased formation of cross-linked envelopes, a marker of keratinocyte terminal differentiation. These effects were prevented by removal of insulin, but not by arsenite addition. We further demonstrate a role for src family kinases in regulation of PKCdelta. Finally, inhibiting epidermal growth factor receptor kinase activity diminished the ability of arsenite to prevent cell enlargement and to suppress insulin-dependent PKCdelta amount and tyrosine 311 phosphorylation. Thus suppression of PKCdelta signaling is a critical feature of arsenite action in preventing keratinocyte differentiation and maintaining proliferative capability.
Figures




Similar articles
-
Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential.Toxicol Appl Pharmacol. 2007 May 15;221(1):119-28. doi: 10.1016/j.taap.2007.02.003. Epub 2007 Feb 14. Toxicol Appl Pharmacol. 2007. PMID: 17400267 Free PMC article.
-
The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation.J Cell Sci. 2006 Feb 1;119(Pt 3):470-81. doi: 10.1242/jcs.02744. Epub 2006 Jan 17. J Cell Sci. 2006. PMID: 16418226
-
Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta.J Biol Chem. 1996 Mar 8;271(10):5325-31. doi: 10.1074/jbc.271.10.5325. J Biol Chem. 1996. PMID: 8621384
-
Methylosome Protein 50 and PKCδ/p38δ Protein Signaling Control Keratinocyte Proliferation via Opposing Effects on p21Cip1 Gene Expression.J Biol Chem. 2015 May 22;290(21):13521-30. doi: 10.1074/jbc.M115.642868. Epub 2015 Apr 7. J Biol Chem. 2015. PMID: 25851901 Free PMC article.
-
Arsenite suppresses Notch1 signaling in human keratinocytes.J Invest Dermatol. 2009 Jan;129(1):155-61. doi: 10.1038/jid.2008.207. Epub 2008 Jul 17. J Invest Dermatol. 2009. PMID: 18633435 Free PMC article.
Cited by
-
Arsenic Stimulates Myoblast Mitochondrial Epidermal Growth Factor Receptor to Impair Myogenesis.Toxicol Sci. 2020 Jul 1;176(1):162-174. doi: 10.1093/toxsci/kfaa031. Toxicol Sci. 2020. PMID: 32159786 Free PMC article.
-
Arsenite suppression of BMP signaling in human keratinocytes.Toxicol Appl Pharmacol. 2013 Jun 15;269(3):290-6. doi: 10.1016/j.taap.2013.02.017. Epub 2013 Apr 6. Toxicol Appl Pharmacol. 2013. PMID: 23566955 Free PMC article.
-
Deducing signaling pathways from parallel actions of arsenite and antimonite in human epidermal keratinocytes.Sci Rep. 2020 Feb 19;10(1):2890. doi: 10.1038/s41598-020-59577-0. Sci Rep. 2020. PMID: 32076005 Free PMC article.
-
Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes.PLoS One. 2015 Feb 3;10(2):e0115341. doi: 10.1371/journal.pone.0115341. eCollection 2015. PLoS One. 2015. PMID: 25647160 Free PMC article.
-
Parallel responses of human epidermal keratinocytes to inorganic SbIII and AsIII.Environ Chem. 2016;13(6):963-970. doi: 10.1071/EN16019. Epub 2016 Apr 26. Environ Chem. 2016. PMID: 28713220 Free PMC article.
References
-
- Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems and methylated arsenic metabolites. Toxicol Sci. 2001;172:249–261. - PubMed
-
- Council NR. Arsenic in Drinking Water. Washington: National Academy Press; 2001.
-
- Tseng C-H. Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis. 2008;199:12–18. - PubMed
-
- Coronado-González JA, Del Razo LM, García-Vargas G, Sanmiguel-Salazar F, Escobedo-de la Peña J. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104:383–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

