Evidence for prostacyclin and cAMP upregulation by bradykinin and insulin-like growth factor 1 in vascular smooth muscle cells
- PMID: 20082561
- PMCID: PMC4327880
- DOI: 10.3109/10799890903563768
Evidence for prostacyclin and cAMP upregulation by bradykinin and insulin-like growth factor 1 in vascular smooth muscle cells
Abstract
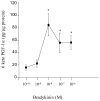
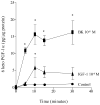
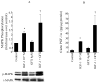
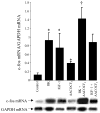
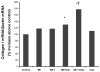
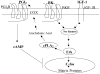
Although bradykinin (BK) and insulin like growth factor-1 (IGF-1) have been shown to modulate the functional and structural integrity of the arterial wall, the cellular mechanisms through which this regulation occurs is still undefined. The present study examined the role of second messenger molecules generated by BK and IGF-1 that could ultimately result in proliferative or antiproliferative signals in vascular smooth muscle cells (VSMC). Activation of BK or IGF-1 receptors stimulated the synthesis and release of prostacyclin (PGI(2)) leading to increased production of cAMP in VSMC. Inhibition of p42/p44(mapk) or src kinases prevented the increase in PGI(2) and cAMP observed in response to BK or IGF-1, indicating a role for these kinases in the regulation of cPLA(2) activity in the VSMC. Inhibition of PKC failed to alter production of PGI(2) in response to BK, but further increased both p42/p44(mapk) activation and the synthesis of PGI(2) produced in response to IGF-1. In addition, both BK and IGF-1 significantly induced the expression of c-fos mRNA levels in VSMC, and this effect of BK was accentuated in the presence a cPLA(2) inhibitor. Finally, inhibition of cPLA(2) activity and/or cyclooxygenase activity enhanced the expression of collagen I mRNA levels in response to BK and IGF-1 stimulation. These findings indicate that the effect of BK or IGF-1 to stimulate VSMC growth is an integrated response to the activation of multiple signaling pathways. Thus, the excessive cell growth that occurs in certain forms of vascular disease could reflect dysfunction in one or more of these pathways.
Figures

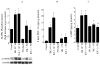
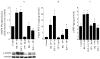
Similar articles
-
Bradykinin-induced p42/p44 MAPK phosphorylation and cell proliferation via Src, EGF receptors, and PI3-K/Akt in vascular smooth muscle cells.J Cell Physiol. 2005 Jun;203(3):538-46. doi: 10.1002/jcp.20250. J Cell Physiol. 2005. PMID: 15573401
-
Simvastatin potenciates PGI(2) release induced by HDL in human VSMC: effect on Cox-2 up-regulation and MAPK signalling pathways activated by HDL.Atherosclerosis. 2004 Jun;174(2):305-13. doi: 10.1016/j.atherosclerosis.2004.01.037. Atherosclerosis. 2004. PMID: 15136060
-
Mechanisms by which bradykinin promotes fibrosis in vascular smooth muscle cells: role of TGF-beta and MAPK.Am J Physiol Heart Circ Physiol. 2000 Dec;279(6):H2829-37. doi: 10.1152/ajpheart.2000.279.6.H2829. Am J Physiol Heart Circ Physiol. 2000. PMID: 11087238
-
Mechanisms through which bradykinin promotes glomerular injury in diabetes.Am J Physiol Renal Physiol. 2005 Mar;288(3):F483-92. doi: 10.1152/ajprenal.00165.2004. Am J Physiol Renal Physiol. 2005. PMID: 15692059
-
Role of reactive oxygen species in bradykinin-induced mitogen-activated protein kinase and c-fos induction in vascular cells.Hypertension. 2000 Apr;35(4):942-7. doi: 10.1161/01.hyp.35.4.942. Hypertension. 2000. PMID: 10775566
Cited by
-
Novel mechanism of plasma prekallikrein (PK) activation by vascular smooth muscle cells: evidence of the presence of PK activator.J Biol Regul Homeost Agents. 2014 Oct-Dec;28(4):587-603. J Biol Regul Homeost Agents. 2014. PMID: 25620170 Free PMC article.
-
The signaling landscape of insulin-like growth factor 1.J Biol Chem. 2025 Jan;301(1):108047. doi: 10.1016/j.jbc.2024.108047. Epub 2024 Dec 3. J Biol Chem. 2025. PMID: 39638246 Free PMC article. Review.
-
The anticoagulant effect of PGI2S and tPA in transgenic umbilical vein endothelial cells is linked to up-regulation of PKA and PKC.Int J Mol Sci. 2014 Feb 19;15(2):2826-39. doi: 10.3390/ijms15022826. Int J Mol Sci. 2014. PMID: 24557578 Free PMC article.
-
Studying GPCR/cAMP pharmacology from the perspective of cellular structure.Front Pharmacol. 2015 Jul 17;6:148. doi: 10.3389/fphar.2015.00148. eCollection 2015. Front Pharmacol. 2015. PMID: 26236239 Free PMC article. Review.
References
-
- Jackson CL, Schwartz SM. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713–736. - PubMed
-
- Clowes AW, Reidy MA, Clowes MA. Kinetics of cellular proliferation after arterial injury. Lab Invest. 1983;49:327–333. - PubMed
-
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. - PubMed
-
- Daeman MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991;68:450–456. - PubMed
-
- Rivard A, Andres V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histology & Histopathology. 2000;15(2):557–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
