Mapping the high-affinity binding domain of 5-substituted benzimidazoles to the proximal N-terminus of the GluN2B subunit of the NMDA receptor
- PMID: 20082612
- PMCID: PMC2825366
- DOI: 10.1111/j.1476-5381.2009.00549.x
Mapping the high-affinity binding domain of 5-substituted benzimidazoles to the proximal N-terminus of the GluN2B subunit of the NMDA receptor
Abstract
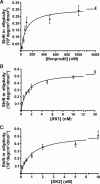
Background and purpose: N-methyl-D-aspartate (NMDA) receptors represent an attractive drug target for the treatment of neurological and neurodegenerative disorders associated with glutamate-induced excitotoxicity. The aim of this study was to map the binding domain of high affinity 5-substituted benzimidazole derivatives [N-{2-[(4-benzylpiperidin-1-yl)methyl]benzimidazol-5-yl}methanesulphonamide (XK1) and N-[2-(4-phenoxybenzyl)benzimidazol-5-yl]methanesulphonamide (XK2)] on the GluN2B subunit of the NMDA receptor.
Experimental approach: The pharmacological antagonistic profiles of XK1 and XK2 were assessed using in vitro rat primary cerebrocortical neurones and two-electrode voltage clamp on Xenopus oocytes expressing heterologous GluN1/GluN2B receptors. Direct ligand binding was determined using the recombinant amino-terminal domain (ATD) of GluN2B.
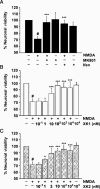
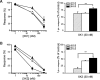
Key results: XK1 and XK2 effectively protected against NMDA-induced excitotoxicity in rat primary cortical neurones. Low concentrations of XK1 (10 nM) and XK2 (1 nM) significantly reversed neuronal death. Both compounds failed to inhibit currents measured from oocytes heterologously expressing GluN1-1a subunit co-assembled with the ATD-deleted GluN2B subunit. XK1 and XK2 showed specific binding to recombinant protein of GluN2B ATD with low nanomolar affinities. Several residues in the recombinant ATD of GluN2B were identified to be critical for conferring XK1 and XK2 sensitivity. The inhibitory effects of XK1 and XK2 were pH-sensitive, being increased at acidic pH.
Conclusions and implications: These results demonstrate that XK1 and XK2 are effective neuroprotective agents in vitro and indicate that 5-substituted benzimidazole derivatives inhibit GluN1/GluN2B receptors via direct binding to the ATD of the GluN2B subunit. These compounds represent valuable alternatives to the classical antagonist ifenprodil as pharmacological tools for studying GluN2B-containing NMDA receptors.
Figures


Similar articles
-
Direct pharmacological monitoring of the developmental switch in NMDA receptor subunit composition using TCN 213, a GluN2A-selective, glycine-dependent antagonist.Br J Pharmacol. 2012 Jun;166(3):924-37. doi: 10.1111/j.1476-5381.2011.01748.x. Br J Pharmacol. 2012. PMID: 22022974 Free PMC article.
-
Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors.Nature. 2011 Jun 15;475(7355):249-53. doi: 10.1038/nature10180. Nature. 2011. PMID: 21677647 Free PMC article.
-
Multiple domains in the C-terminus of NMDA receptor GluN2B subunit contribute to neuronal death following in vitro ischemia.Neurobiol Dis. 2016 May;89:223-34. doi: 10.1016/j.nbd.2015.11.007. Epub 2015 Nov 12. Neurobiol Dis. 2016. PMID: 26581639
-
GluN2B subunit selective N-methyl-D-aspartate receptor ligands: Democratizing recent progress to assist the development of novel neurotherapeutics.Mol Divers. 2024 Jun;28(3):1765-1792. doi: 10.1007/s11030-023-10656-0. Epub 2023 Jun 2. Mol Divers. 2024. PMID: 37266849 Free PMC article. Review.
-
NMDA Receptor Antagonists: Emerging Insights into Molecular Mechanisms and Clinical Applications in Neurological Disorders.Pharmaceuticals (Basel). 2024 May 15;17(5):639. doi: 10.3390/ph17050639. Pharmaceuticals (Basel). 2024. PMID: 38794209 Free PMC article. Review.
Cited by
-
Cleavage of the NR2B subunit amino terminus of N-methyl-D-aspartate (NMDA) receptor by tissue plasminogen activator: identification of the cleavage site and characterization of ifenprodil and glycine affinities on truncated NMDA receptor.J Biol Chem. 2012 Jul 20;287(30):25520-9. doi: 10.1074/jbc.M112.374397. Epub 2012 May 18. J Biol Chem. 2012. PMID: 22610100 Free PMC article.
References
-
- Birmingham K. Future of neuroprotective drugs in doubt. Nat Med. 2002;8:5. - PubMed
-
- Borza I, Domány G. NR2B selective NMDA antagonists: the evolution of the ifenprodil-type pharmacophore. Curr Top Med Chem. 2006;6:687–695. - PubMed
-
- Carter C, Benavides J, Legendre P, Vincent JD, Noel F, Thuret F, et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II. Evidence for N-methyl-D-aspartate receptor antagonist properties. J Pharmacol Exp Ther. 1988;247:1222–1232. - PubMed
-
- Cheung NS, Pascoe CJ, Giardina SF, John CA, Beart PM. Micromolar L-glutamate induces extensive apoptosis in an apoptotic-necrotic continuum of insult-dependent, excitotoxic injury in cultured cortical neurons. Neuropharmacology. 1998;37:1419–1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

