Early defect of transforming growth factor β1 formation in Huntington's disease
- PMID: 20082658
- PMCID: PMC3922377
- DOI: 10.1111/j.1582-4934.2010.01011.x
Early defect of transforming growth factor β1 formation in Huntington's disease
Abstract
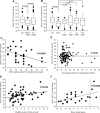
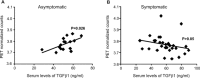
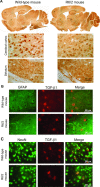
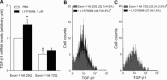
A defective expression or activity of neurotrophic factors, such as brain- and glial-derived neurotrophic factors, contributes to neuronal damage in Huntington's disease (HD). Here, we focused on transforming growth factor-β (TGF-β(1) ), a pleiotropic cytokine with an established role in mechanisms of neuroprotection. Asymptomatic HD patients showed a reduction in TGF-β(1) levels in the peripheral blood, which was related to trinucleotide mutation length and glucose hypometabolism in the caudate nucleus. Immunohistochemical analysis in post-mortem brain tissues showed that TGF-β(1) was reduced in cortical neurons of asymptomatic and symptomatic HD patients. Both YAC128 and R6/2 HD mutant mice showed a reduced expression of TGF-β(1) in the cerebral cortex, localized in neurons, but not in astrocytes. We examined the pharmacological regulation of TGF-β(1) formation in asymptomatic R6/2 mice, where blood TGF-β(1) levels were also reduced. In these R6/2 mice, both the mGlu2/3 metabotropic glutamate receptor agonist, LY379268, and riluzole failed to increase TGF-β(1) formation in the cerebral cortex and corpus striatum, suggesting that a defect in the regulation of TGF-β(1) production is associated with HD. Accordingly, reduced TGF-β(1) mRNA and protein levels were found in cultured astrocytes transfected with mutated exon 1 of the human huntingtin gene, and in striatal knock-in cell lines expressing full-length huntingtin with an expanded glutamine repeat. Taken together, our data suggest that serum TGF-β(1) levels are potential biomarkers of HD development during the asymptomatic phase of the disease, and raise the possibility that strategies aimed at rescuing TGF-β(1) levels in the brain may influence the progression of HD.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Sathasivam K, Hobbs C, Turmaine M, et al. Formation of polyglutamine inclusions in non-CNS tissue. Hum Mol Genet. 1999;8:813–22. - PubMed
-
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. - PubMed
-
- Vonsattel JP, Myers RH, Stevens TJ, et al. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–77. - PubMed
-
- de la Monte SM, Vonsattel JP Richardson EPJr. Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. J Neuropathol Exp Neurol. 1988;47:516–25. - PubMed
-
- Beal MF, Kowall NW, Ellison DW, et al. Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid. Nature. 1986;321:168–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

