Characterization of differential properties of rabbit tendon stem cells and tenocytes
- PMID: 20082706
- PMCID: PMC2822826
- DOI: 10.1186/1471-2474-11-10
Characterization of differential properties of rabbit tendon stem cells and tenocytes
Abstract
Background: Tendons are traditionally thought to consist of tenocytes only, the resident cells of tendons; however, a recent study has demonstrated that human and mouse tendons also contain stem cells, referred to as tendon stem/progenitor cells (TSCs). However, the differential properties of TSCs and tenocytes remain largely undefined. This study aims to characterize the properties of these tendon cells derived from rabbits.
Methods: TSCs and tenocytes were isolated from patellar and Achilles tendons of rabbits. The differentiation potential and cell marker expression of the two types of cells were examined using histochemical, immunohistochemical, and qRT-PCR analysis as well as in vivo implantation. In addition, morphology, colony formation, and proliferation of TSCs and tenocytes were also compared.
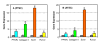
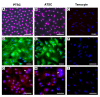
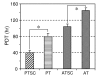
Results: It was found that TSCs were able to differentiate into adipocytes, chondrocytes, and osteocytes in vitro, and form tendon-like, cartilage-like, and bone-like tissues in vivo. In contrast, tenocytes had little such differentiation potential. Moreover, TSCs expressed the stem cell markers Oct-4, SSEA-4, and nucleostemin, whereas tenocytes expressed none of these markers. Morphologically, TSCs possessed smaller cell bodies and larger nuclei than ordinary tenocytes and had cobblestone-like morphology in confluent culture whereas tenocytes were highly elongated. TSCs also proliferated more quickly than tenocytes in culture. Additionally, TSCs from patellar tendons formed more numerous and larger colonies and proliferated more rapidly than TSCs from Achilles tendons.
Conclusions: TSCs exhibit distinct properties compared to tenocytes, including differences in cell marker expression, proliferative and differentiation potential, and cell morphology in culture. Future research should investigate the mechanobiology of TSCs and explore the possibility of using TSCs to more effectively repair or regenerate injured tendons.
Figures




Similar articles
-
The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo.Biomaterials. 2011 Oct;32(29):6972-81. doi: 10.1016/j.biomaterials.2011.05.088. Epub 2011 Jun 23. Biomaterials. 2011. PMID: 21703682 Free PMC article.
-
The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries.Stem Cell Res Ther. 2015 Sep 15;6(1):173. doi: 10.1186/s13287-015-0172-4. Stem Cell Res Ther. 2015. PMID: 26373929 Free PMC article.
-
Tendon Stem Cells: Mechanobiology and Development of Tendinopathy.Adv Exp Med Biol. 2016;920:53-62. doi: 10.1007/978-3-319-33943-6_5. Adv Exp Med Biol. 2016. PMID: 27535248 Review.
-
Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy.J Orthop Res. 2010 May;28(5):639-43. doi: 10.1002/jor.21046. J Orthop Res. 2010. PMID: 19918904
-
Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements.J Hand Ther. 2012 Apr-Jun;25(2):133-40; quiz 141. doi: 10.1016/j.jht.2011.07.004. Epub 2011 Sep 17. J Hand Ther. 2012. PMID: 21925835 Free PMC article. Review.
Cited by
-
Mesenchymal stem cell applications to tendon healing.Muscles Ligaments Tendons J. 2012 Oct 16;2(3):222-9. Print 2012 Jul. Muscles Ligaments Tendons J. 2012. PMID: 23738300 Free PMC article.
-
Therapeutic Roles of Tendon Stem/Progenitor Cells in Tendinopathy.Stem Cells Int. 2016;2016:4076578. doi: 10.1155/2016/4076578. Epub 2016 Apr 19. Stem Cells Int. 2016. PMID: 27195010 Free PMC article. Review.
-
Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model.Tissue Eng Part A. 2013 Nov;19(21-22):2439-51. doi: 10.1089/ten.TEA.2012.0453. Epub 2013 Aug 9. Tissue Eng Part A. 2013. PMID: 23815150 Free PMC article.
-
Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration.J Orthop Res. 2020 Jan;38(1):7-12. doi: 10.1002/jor.24475. Epub 2019 Sep 30. J Orthop Res. 2020. PMID: 31529731 Free PMC article. Review.
-
Retinoic acid receptor signaling preserves tendon stem cell characteristics and prevents spontaneous differentiation in vitrox.Stem Cell Res Ther. 2016 Mar 22;7:45. doi: 10.1186/s13287-016-0306-3. Stem Cell Res Ther. 2016. PMID: 27001426 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

