Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients
- PMID: 20082712
- PMCID: PMC2819256
- DOI: 10.1186/1742-2094-7-5
Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients
Abstract
Background: Traumatic brain injury (TBI) initiates interrelated inflammatory and coagulation cascades characterized by wide-spread cellular activation, induction of leukocyte and endothelial cell adhesion molecules and release of soluble pro/antiinflammatory cytokines and thrombotic mediators. Resuscitative care is focused on optimizing cerebral perfusion and reducing secondary injury processes. Hypertonic saline is an effective osmotherapeutic agent for the treatment of intracranial hypertension and has immunomodulatory properties that may confer neuroprotection. This study examined the impact of hypertonic fluids on inflammatory/coagulation cascades in isolated head injury.
Methods: Using a prospective, randomized controlled trial we investigated the impact of prehospital resuscitation of severe TBI (GCS < 8) patients using 7.5% hypertonic saline in combination with 6% dextran-70 (HSD) vs 0.9% normal saline (NS), on selected cellular and soluble inflammatory/coagulation markers. Serial blood samples were drawn from 65 patients (30 HSD, 35 NS) at the time of hospital admission and at 12, 24, and 48-h post-resuscitation. Flow cytometry was used to analyze leukocyte cell-surface adhesion (CD62L, CD11b) and degranulation (CD63, CD66b) molecules. Circulating concentrations of soluble (s)L- and sE-selectins (sL-, sE-selectins), vascular and intercellular adhesion molecules (sVCAM-1, sICAM-1), pro/antiinflammatory cytokines [tumor necrosis factor (TNF)-alpha and interleukin (IL-10)], tissue factor (sTF), thrombomodulin (sTM) and D-dimers (D-D) were assessed by enzyme immunoassay. Twenty-five healthy subjects were studied as a control group.
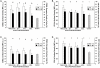
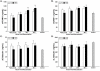
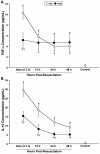
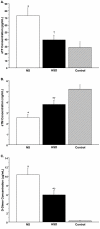
Results: TBI provoked marked alterations in a majority of the inflammatory/coagulation markers assessed in all patients. Relative to control, NS patients showed up to a 2-fold higher surface expression of CD62L, CD11b and CD66b on polymorphonuclear neutrophils (PMNs) and monocytes that persisted for 48-h. HSD blunted the expression of these cell-surface activation/adhesion molecules at all time-points to levels approaching control values. Admission concentrations of endothelial-derived sVCAM-1 and sE-selectin were generally reduced in HSD patients. Circulating sL-selectin levels were significantly elevated at 12 and 48, but not 24 h post-resuscitation with HSD. TNF-alpha and IL-10 levels were elevated above control throughout the study period in all patients, but were reduced in HSD patients. Plasma sTF and D-D levels were also significantly lower in HSD patients, whereas sTM levels remained at control levels.
Conclusions: These findings support an important modulatory role of HSD resuscitation in attenuating the upregulation of leukocyte/endothelial cell proinflammatory/prothrombotic mediators, which may help ameliorate secondary brain injury after TBI.
Trial registration: NCT00878631.
Figures
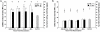
References
-
- Schmidt OI, Infanger M, Heyde CE, Ertel W, Stahel PF. The role of neuroinflammation in traumatic brain injury. Eur J Trauma. 2004;3:135–149.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

